Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 9 (SOLVED)-PART-III
- Explain the principle of electrolytic refining with an example.
Text Solution
|
- How will you prepare phosphine and explain the purification of phosphi...
Text Solution
|
- Justify the position of lanthanides and actinides in the periodic...
Text Solution
|
- Distinguish between isotropy and anisotropy ?
Text Solution
|
- What are the merits and limitations of the intermediate compound theor...
Text Solution
|
- Write a note on sacrificial protection .
Text Solution
|
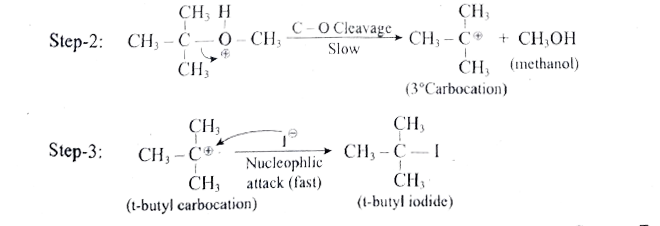
- 1 mole of HI is allowed to react with t-butyl methylether. Identify th...
Text Solution
|
- How will you prove the presence of aldehyde group in glucose ?
Text Solution
|
- Explain about anaesthetics with their types.
Text Solution
|