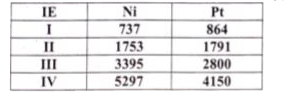
Text Solution
Verified by Experts
Topper's Solved these Questions
TRANSITION AND INNER TRANSITION ELEMENTS
FULL MARKS|Exercise Additional Questions (I. Choose the correct answer)|47 VideosTRANSITION AND INNER TRANSITION ELEMENTS
FULL MARKS|Exercise Additional Questions (II. Fill in the blanks)|31 VideosTRANSITION AND INNER TRANSITION ELEMENTS
FULL MARKS|Exercise Additional Questions (X. 5 Mark Questions.)|4 VideosSURFACE CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS (5 Marks questions)|12 Videos
Similar Questions
Explore conceptually related problems