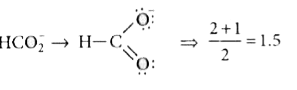
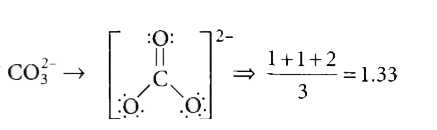
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2014-CHEMISTRY
- The statement that is NOT correct is
Text Solution
|
- The correct arrangement for the ions in the increasing order of their ...
Text Solution
|
- The correct arrangement of the species in the decreasing order of the ...
Text Solution
|
- The species that is not hydrolysed in water is
Text Solution
|
- For the properties mentioned, the correct trend for the different spec...
Text Solution
|
- A correct statement is
Text Solution
|
- Iodoform reaction is answered by all, except
Text Solution
|
- A crystalline solid XY3 has ccp arrangement for its element Y. X occup...
Text Solution
|
- C6H5COOH underset(2.Delta)overset(1.NH3)to P overset(NaOBr)to Q unders...
Text Solution
|
- The statement that is NOT correct is
Text Solution
|
- Match the reactant in column-I with the reaction in column-II
Text Solution
|
- The statement that is NOT correct is
Text Solution
|
- In which one of the pairs of ion given, there is an ion that forms a c...
Text Solution
|
- A crystalline solid X reacts with dil HCI to liberate a gas Y. Y decol...
Text Solution
|
- An aromatic compound 'A'(C7H9N) on reacting with NaNO2 // HCl at 0^(...
Text Solution
|
- The statement that is NOT correct is
Text Solution
|
- A solution of 1.25 g of 'P' in 50 g of water lowers freezing point by ...
Text Solution
|
- Volume occupied by single CsCl ion pair in a crystal is 7.014 xx 10^(-...
Text Solution
|
- For Cr2O7^(2-) + 14 H^(+) + 6e^(-) to 2Cr^(+3) + 7H2O, E^(@) = 1.33 V ...
Text Solution
|
- 1.78 g of an optically active L-amino acid (A) is treated with NaNO2//...
Text Solution
|