A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2017-CHEMISTRY
- The co-ordination number and the oxidation state of the element .M. in...
Text Solution
|
- Which of the following crystals has unit cell such that a ne b ne c an...
Text Solution
|
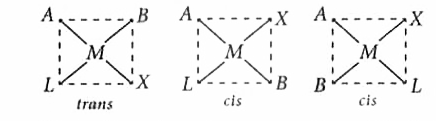
- Square planar complex of the type MAXBL (where A, B, X and L are unide...
Text Solution
|
- Which of the following statements is in accordance with the Arrhenius ...
Text Solution
|
- In a face centred cubic arrangement of A and B atoms in which ‘A’ atom...
Text Solution
|
- The standard reduction potential at 298 K for the following half cell ...
Text Solution
|
- Which of the following reagents cannot be used to oxidize primary alco...
Text Solution
|
- In the following sequence of reactions CH3Br overset(KCN) rarrA ove...
Text Solution
|
- When the pure solvent diffuses out of the solution through the semi-pe...
Text Solution
|
- The metal extracted by leaching with a cyanide
Text Solution
|
- On addition of mineral acid to an aqueous solution of Borax, the follo...
Text Solution
|
- According to crystal field theory, the M-L bond in a complex is
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- The Glycosidic linkage present in sucrose is between
Text Solution
|
- Pick the wrong statement from the following:
Text Solution
|
- Which of the following elements forms p(pi)-p(pi) bond with itself?
Text Solution
|
- Which one of the following noble gases has an unusual property of diff...
Text Solution
|
- The Van't Hoff factor (i) accounts for
Text Solution
|
- The pressure of real gases is less than that of ideal gas because of
Text Solution
|
- The reaction quotient ‘Q.’ is useful in predicting the direction of th...
Text Solution
|