A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2018-CHEMISTRY
- Which of the following is the correct order of radius?
Text Solution
|
- The intramolecular hydrogen bond is present in
Text Solution
|
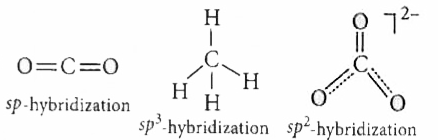
- The state of hybrid orbitals of carbon in CO(2), CH(4) and CO(3)^(2-)...
Text Solution
|
- For an ideal gas, compressibility factor is
Text Solution
|
- The relationship between K(p) and K(c ) is K(p) = K(c ) (RT) Delta n...
Text Solution
|
- Acidity of BF, can be explained on which of the following concepts ?
Text Solution
|
- For the redox reaction x MnO(4)^(-) + yH(2)C(2)O(4) + zH^(+) to m M...
Text Solution
|
- H2O2 is
Text Solution
|
- Dead burnt plaster is
Text Solution
|
- Identify the following compound which exhibits geometrical isomerism:
Text Solution
|
- During the fusion of organic compound with sodium metal, nitrogen pre...
Text Solution
|
- The reagent ' X ' used for the following reaction is R - C -= C- R'+...
Text Solution
|
- Which of the following ions will cause hardness in water ?
Text Solution
|
- Which of the following oxides shows electrical properties like metals?
Text Solution
|
- Which of the following aqueous solutions should have the highest boili...
Text Solution
|
- The charge required for the reduction of 1 mole of MnO(4)^(-) to MnO(...
Text Solution
|
- For the reaction , 2SO(2) +O(2) hArr 2SO(3), the rate of disappearan...
Text Solution
|
- Which of the following electrolytes will have maximum coagulating val...
Text Solution
|
- Electrolytic refining is used to purify which of the following metal...
Text Solution
|
- Dry ice is
Text Solution
|