Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Set - 1 Comptt.
- Explain the following terms with suitable example: a. Schottky defec...
Text Solution
|
- 45 g of ethylene glycol C(2)H(6)O(2) is mixed with 600 g of water. Cal...
Text Solution
|
- The rate constant of a reaction at 500 K and 700 K are 0.02s^(-1), res...
Text Solution
|
- Define the following terms : (i) Electrophoresis (ii) Adsorption ...
Text Solution
|
- Outline the principels of refining of metals by the following methods ...
Text Solution
|
- Write down the reactions taking place in different zones in the blast ...
Text Solution
|
- What is lanthanoid contraction? What are the consequences of lanthanol...
Text Solution
|
- Indicate the types of isomerism exhibited by the following complexes :...
Text Solution
|
- Name the following according to IUPAC systems : (i) CH3-underset(OH)...
Text Solution
|
- How are the following conversions carried out ? (i) Propene to propa...
Text Solution
|
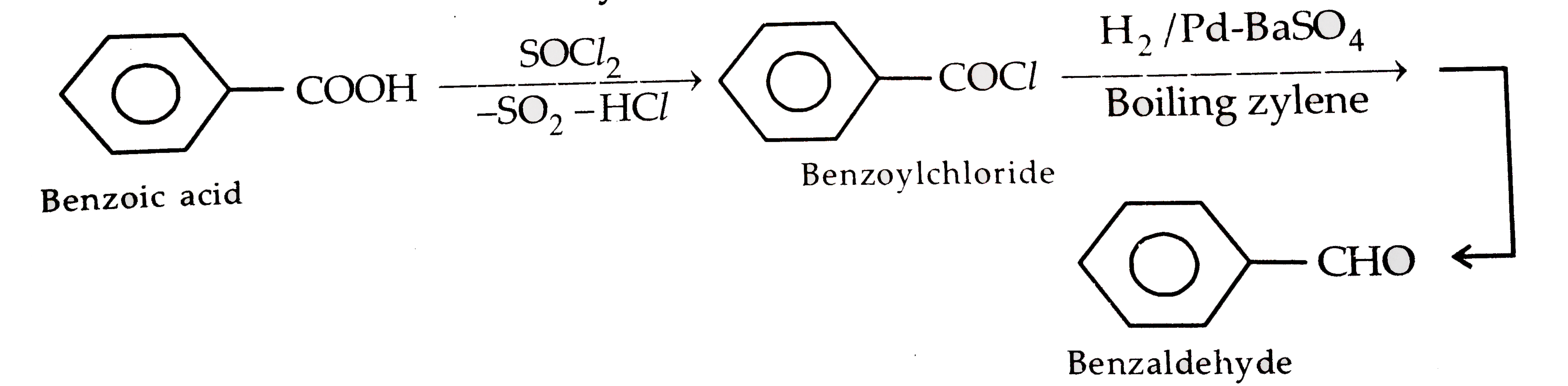
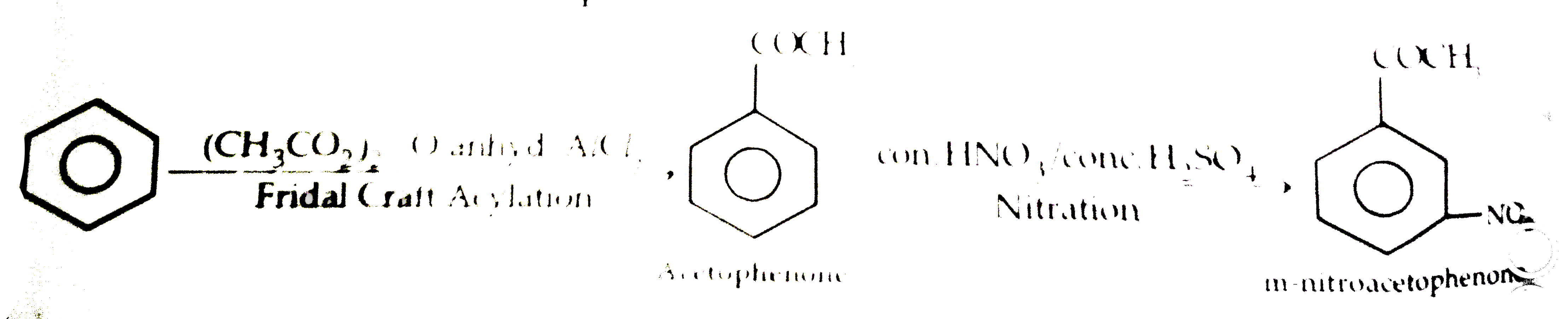
- An aromatic compound (A) on treatment with aqueous ammonia and heating...
Text Solution
|
- How are vitamins classified? Name the vitamin responsible for the coag...
Text Solution
|
- Write the names and structures of the monomers of the following polyer...
Text Solution
|
- Ramesh went to a department store to purchase groceties. On one of she...
Text Solution
|
- Define the following terms : (i) Molar conductivity (wedge(m)) (ii)...
Text Solution
|
- Define the term degree of dissociation. Write an expression that relat...
Text Solution
|
- (a) Complete the followingh chemical reaction equations : (i) Cu+HNO...
Text Solution
|
- (a) Write balanced equations for the following reactions : (i) Chlor...
Text Solution
|
- Describe the following giving chemical equations : (i) De-carboxylat...
Text Solution
|
- (a) Describe the following actions : (i) Acetylation (ii) Aldol co...
Text Solution
|