Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-General Instrucations
- Why is adsorption always exothermic ?
Text Solution
|
- Predict the major product formed when sodium ethoxide reacts with tert...
Text Solution
|
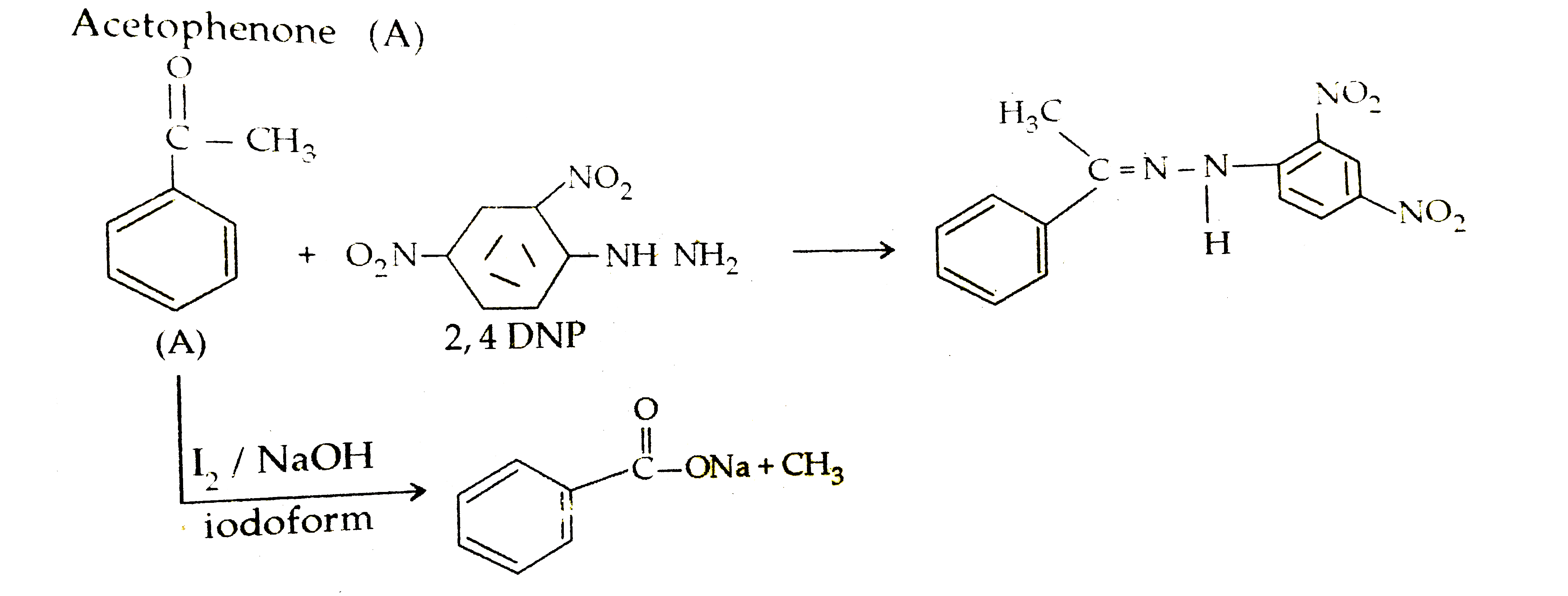
- An aromatic organic compound 'A' with formula C8H8O gives positive DNP...
Text Solution
|
- For the reaction AtoB, the rate of reaction becomes three times when t...
Text Solution
|
- Write the coordination isomer of [Cu(NH3)4][PtCl4].
Text Solution
|
- A current of 1.50 A was passed through an electrolytic cell containin...
Text Solution
|
- The conductivity of a 0.01 M solution of acetic acid at 298 k is 1.65...
Text Solution
|
- Draw the structure of the following : (i) XeF2 (ii) BrF5
Text Solution
|
- Which one of the following compounds is more reactive towards S(N)2 re...
Text Solution
|
- Identify the following : (i) Transition metal of 3d series that exhi...
Text Solution
|
- Why a mixture of carbon disulphide and acetone shows positive deviatio...
Text Solution
|
- Consider the following reaction : Cu(s)+2Ag^(+)(aq)to 2Ag(s) +Cu^(2+)(...
Text Solution
|
- Write the role of : (i) NaCn in the extraction of gold from its ore....
Text Solution
|
- (i) Complete the following equations : (a) 2MnO(4)^(-)+ 5SO(3)^(2-)+...
Text Solution
|
- Write the preparation of following : (i) KMnO4 from K2MnO4 (ii) Na...
Text Solution
|
- Do as directed : (i) Arrange the following compounds in the increasi...
Text Solution
|
- Give the formula of monomers involved in the formation of the followin...
Text Solution
|
- Write IUPAC name for each of the following complexes : (i) [Ni(NH3)6...
Text Solution
|
- (i) Complete the following reaction and suggest a suitable mechanism f...
Text Solution
|
- Explain the following : (i) Amino acids behave like salts rather tha...
Text Solution
|