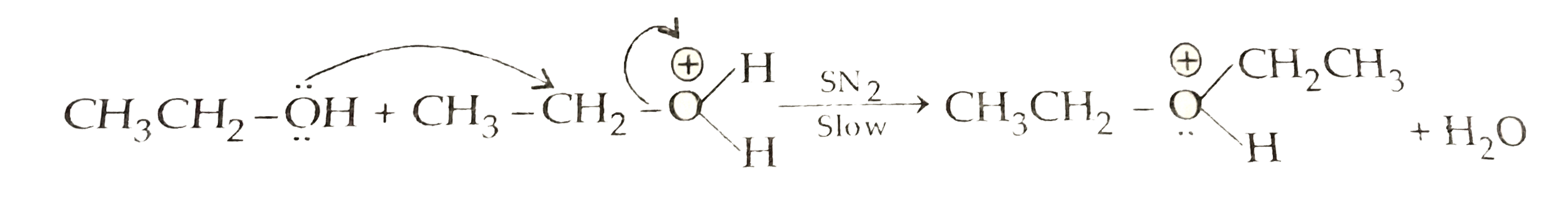
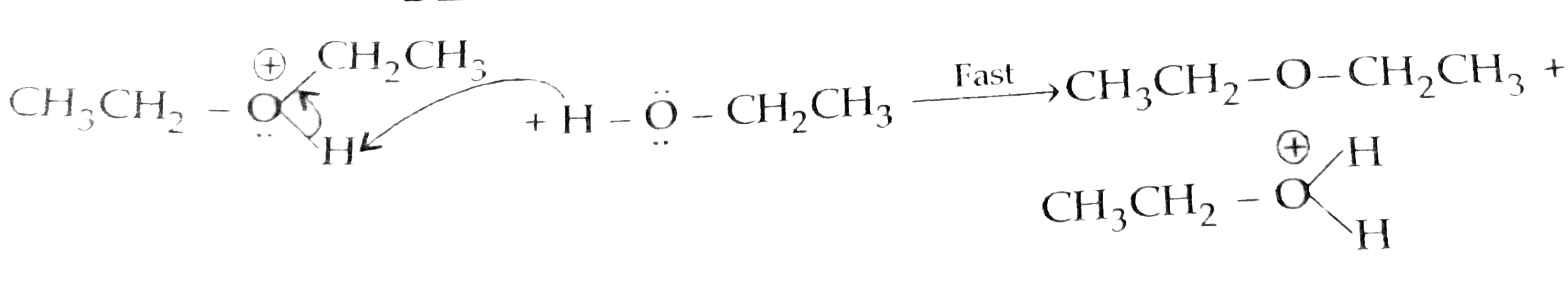
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Set I
- A 10% solution (by mass) of sucrose in water has freezing point of 26...
Text Solution
|
- (a) Calculate the mass of Ag deposited at cathode when a current of 2 ...
Text Solution
|
- (i) What type of isomerism is shown by the complex [Co(NH(3))(6)] [Cr(...
Text Solution
|
- Write one difference in each of the following: (i) Lyophobic sol and...
Text Solution
|
- Following data are obtained for the reaction: N(2)O(5) rarr 2NO(2) +...
Text Solution
|
- Following compounds are given to you : 2-Bromopentane, 2-Bromo-2-met...
Text Solution
|
- (a) Write the principle of method used for the refining of germanium ...
Text Solution
|
- Write structures of compounds A,B and C in each of the following react...
Text Solution
|
- Do the following conversions in not more than two steps: (i) Benzoic...
Text Solution
|
- Write the structure of the monomers used for getting the following pol...
Text Solution
|
- Define the following : (i) Anionic detergents (ii) Broad spectrum ...
Text Solution
|
- Given reasion: (i) Thermal stability decreases from H(2)O " to " H(2...
Text Solution
|
- Give reasons for the following (a) Acetylation of aniline reduces it...
Text Solution
|
- After wathicng a programme on TV about te presence of carcinogens (can...
Text Solution
|
- (a) Account for the following : (i) Transition metals form large num...
Text Solution
|
- (a) (i) How is the variability in oxidation states of transition metal...
Text Solution
|
- (a) An element has atomic mass 93 g mol^(1) and density 11.5 g cm^(-3)...
Text Solution
|
- Calculate the number of unit cells in 8.1g of aluminium if it crystall...
Text Solution
|
- (a) Write the product(s) in the following reactions (i) (ii) CH(3...
Text Solution
|
- (a) Write the formula of reagents used in the following reactions: (...
Text Solution
|