Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARDS PREVIOUS YEAR|Exercise QUESTION PAPER (SECTION-C)|22 VideosXII BOARDS
XII BOARDS PREVIOUS YEAR|Exercise IMPORTANT QUESTIONS FOR PRACTICE CHEMISTRY (THEORY-XII) UNSOLVED)|42 VideosXII BOARDS
XII BOARDS PREVIOUS YEAR|Exercise QUESTION PAPER (SECTION-A)|7 VideosSAMPLE PAPER 2019
XII BOARDS PREVIOUS YEAR|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-QUESTION PAPER (SECTION-B)
- What is the freezing point of a solution containing 8.1g Br in 100g wa...
Text Solution
|
- Calculate the molality of ethanol solution in which the mole fraction ...
Text Solution
|
- Identify the reaction and write the IUPAC name of the product formed :...
Text Solution
|
- Write the structures of the cross-aldol products between ethanal and p...
Text Solution
|
- What is the role of tertiary-butyl peroxide in the polymerisation alke...
Text Solution
|
- Write the structures of the monomers of the following polymers :
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
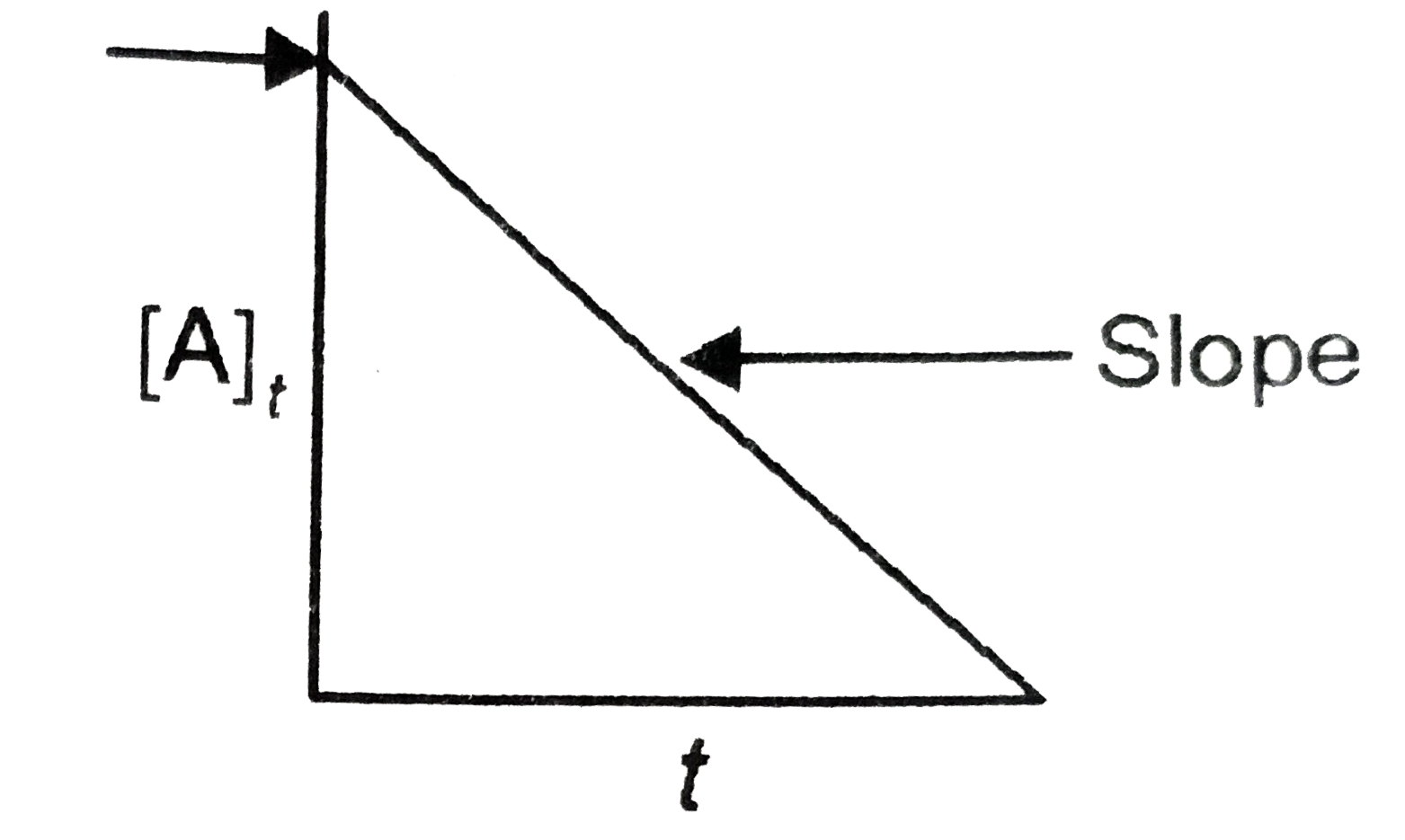
- For a certain chemical reaction variation in concentration [A] vs. tim...
Text Solution
|
- Draw the molecular structures of the following : (a) Noble gas spec...
Text Solution
|
- (i) On the basis of the standard electrode potential values stated for...
Text Solution
|