Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-I: Subjective Question Section (F)|3 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-I: Subjective Question Section (G)|4 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-I: Subjective Question Section (D)|2 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Exercise Part-I: Subjective Question Section (E)
- For the complex K(2)[Cr(NO)(NH(3))(CN)(4)],mu=1.73 BM. (i) Write IUP...
Text Solution
|
- Predict the hybridisation and geometry of the following complexes. ...
Text Solution
|
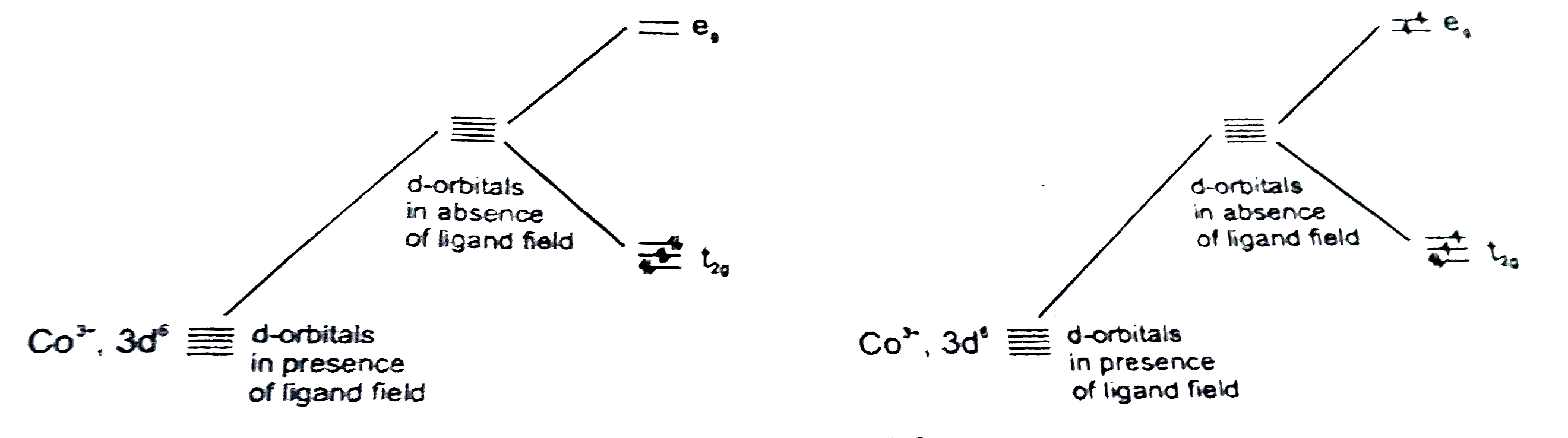
- [Co(NH(3))(6)]^(3+) & [CoF(6)]^(3-) both are complexes of Co(III), but...
Text Solution
|
- Arrange the following in increasing order as directed. (a) (i) [CoC...
Text Solution
|
- For each of the following complexes, draw a crystal field energy-level...
Text Solution
|