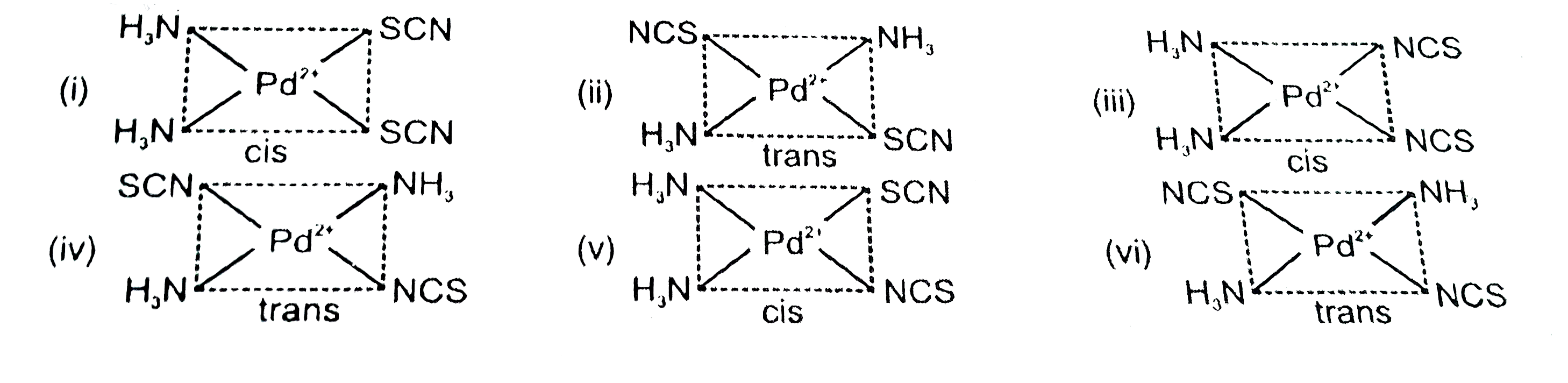
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-I: Subjective Question Section (H)|2 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-II: Section (A)|9 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise Part-I: Subjective Question Section (F)|3 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Exercise Part-I: Subjective Question Section (G)
- What type of isomers are the following : (i) [Mn(CO)(5)SCN] and [Mn...
Text Solution
|
- (a) Draw all possible constitutional isomers of the compound Ru(NH(3))...
Text Solution
|
- How many geomatrical isomers are possible for each of the following co...
Text Solution
|
- Which of the following complexes can exists as enantiomers ? Draw thei...
Text Solution
|