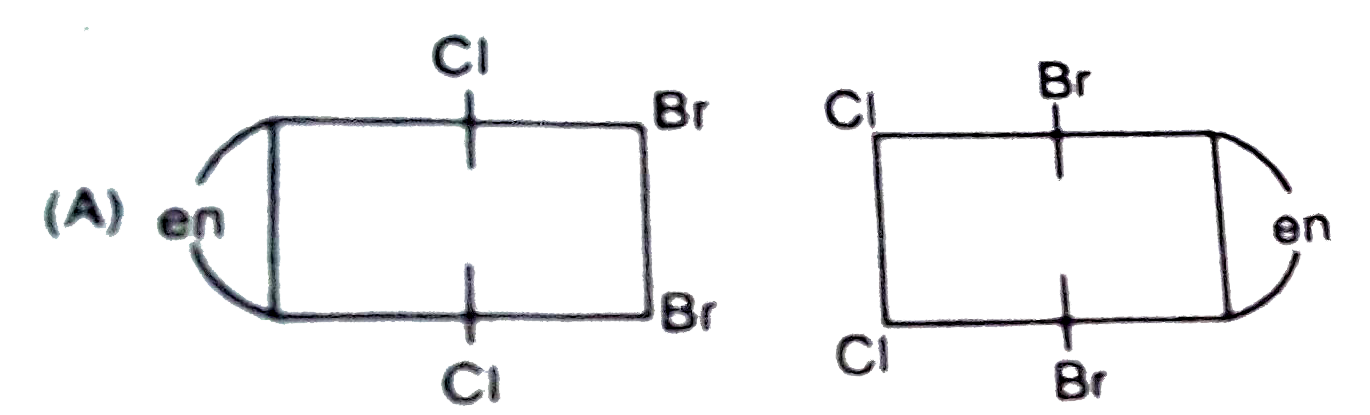
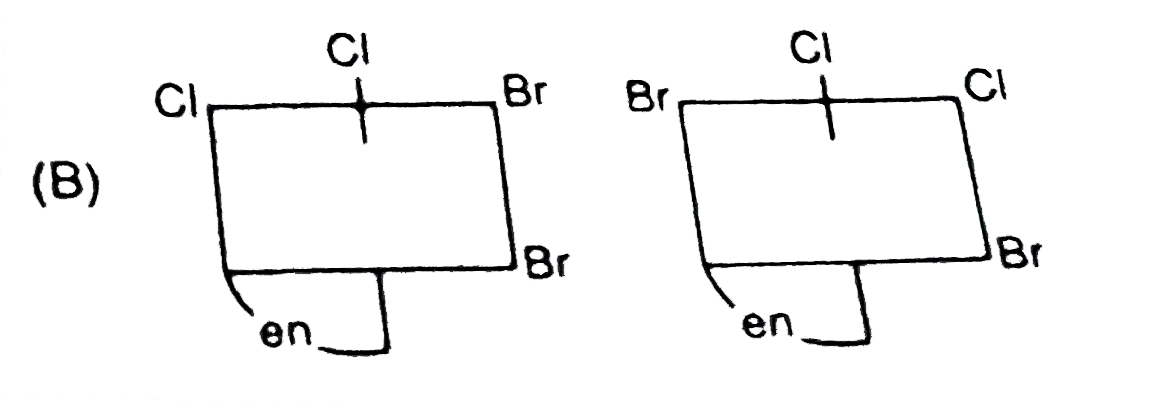
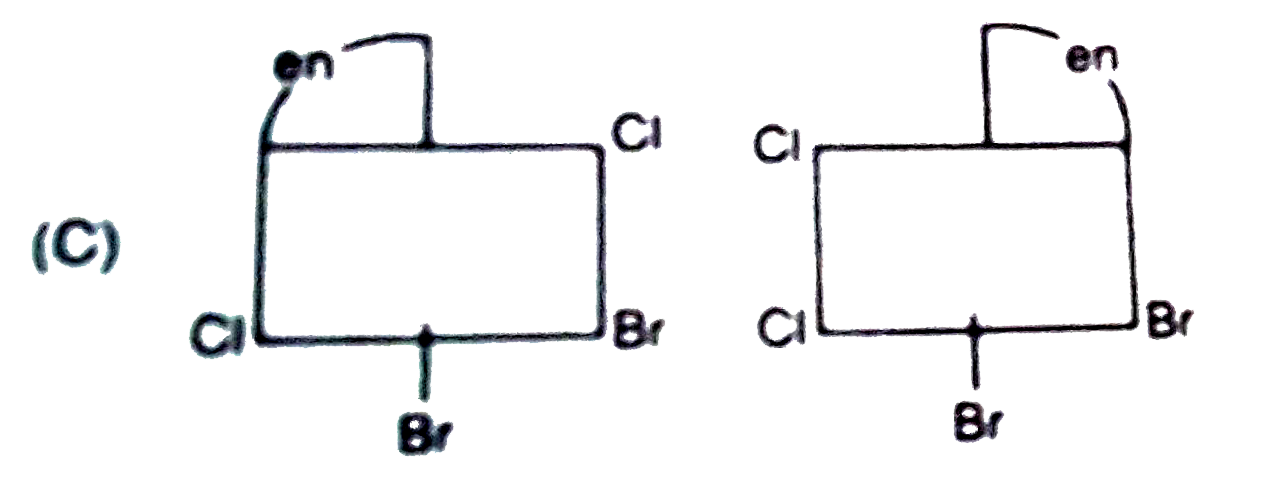
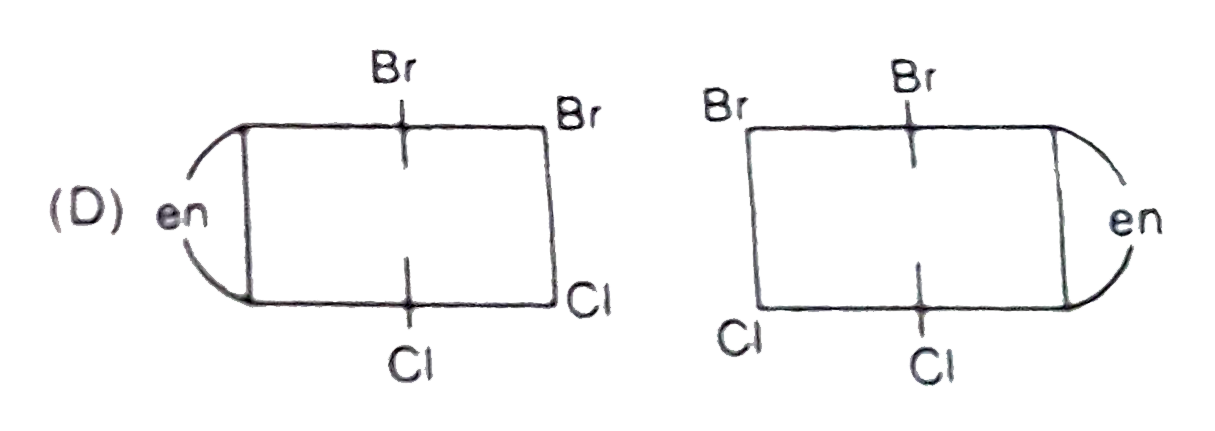
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Subjected Question|7 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Only one option correct type|21 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-I|30 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-II
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|
- When any solution passess through a cation exchange resin that is in a...
Text Solution
|
- A person having osteoporosis is suffering from lead poisoning. Ethlene...
Text Solution
|
- Consider the following reaction and statements: [Co(NH(3))(4)Br(2)]^...
Text Solution
|
- The complex that shows optical acitivity is
Text Solution
|
- For [FeF(6)]^(3-) and [CoF(6)]^(3-), the statement that is correct is ...
Text Solution
|
- Which of the following statementss about ammonium cerium (IV) nitrate,...
Text Solution
|
- Which one of the following reactions is correct ?
Text Solution
|
- How many isomers are possible for complex [Co("ox")(2)Cl(2)]^(+) ?
Text Solution
|
- In which of the following complexes the metal ion has the lowest ionic...
Text Solution
|
- Which of the complexes has the magnetic moment of 3.87 BM ?
Text Solution
|
- IUPAC name of complex ion [CrCl(2)(ox)(2)]^(3-) is
Text Solution
|
- The type of isomerism that Co(NH(3))(4)Br(2)Cl(2) can exhibit is/are
Text Solution
|
- Metal 'M' forms as carbonyl compound in which it is present in its low...
Text Solution
|
- An appropriate reagent for the conversion of 1-propanol to 1-propanal ...
Text Solution
|
- The complex ion that does not have d electrons in the metal atom is
Text Solution
|
- The complex ion . [M(en)Br(2)I(2)]^(-1), has two optical isomers. Thei...
Text Solution
|
- The IUPAC name of the complex [PT(en)(NH(3))(cl)(2)(ONO)][Ag(CN)(2)] i...
Text Solution
|
- The C-O bond length is the shortest in:
Text Solution
|
- The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-)...
Text Solution
|