A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE|Exercise Part - II NSEC|31 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise Part - III Practice Test-2|1 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise JEE MAIN (Online Problem)|16 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-AROMATIC COMPOUNDS-APSP (Part-I)
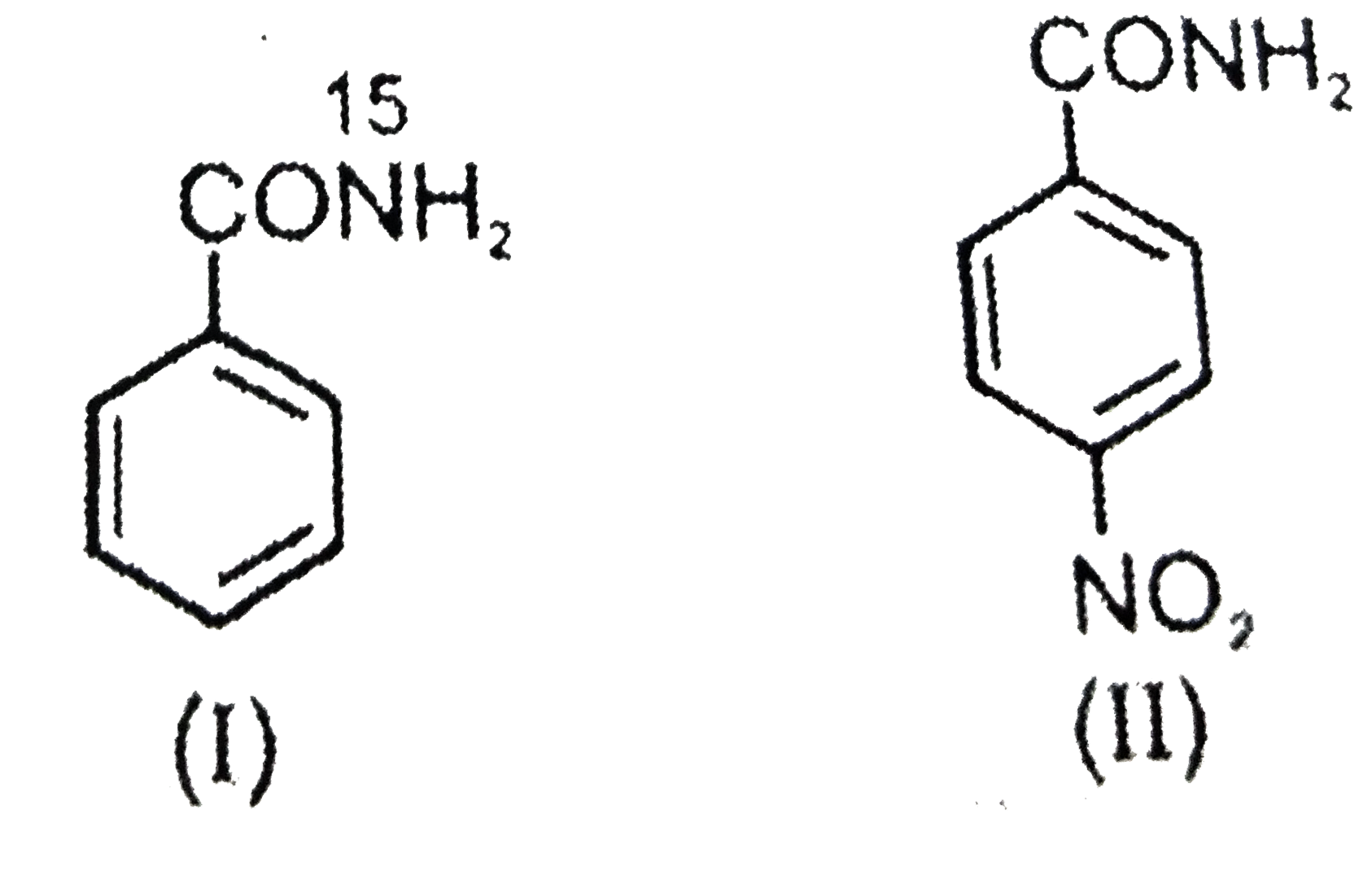
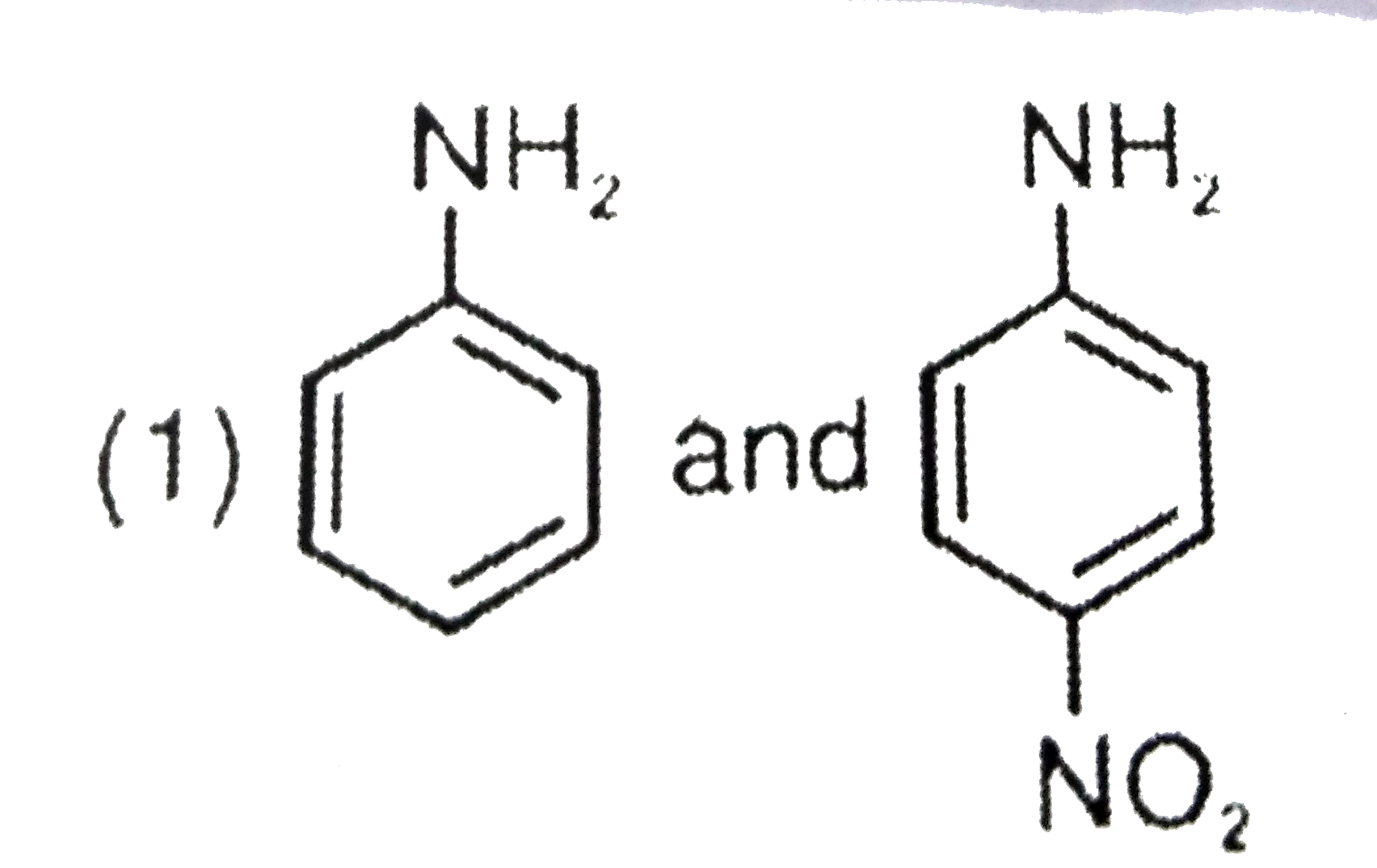
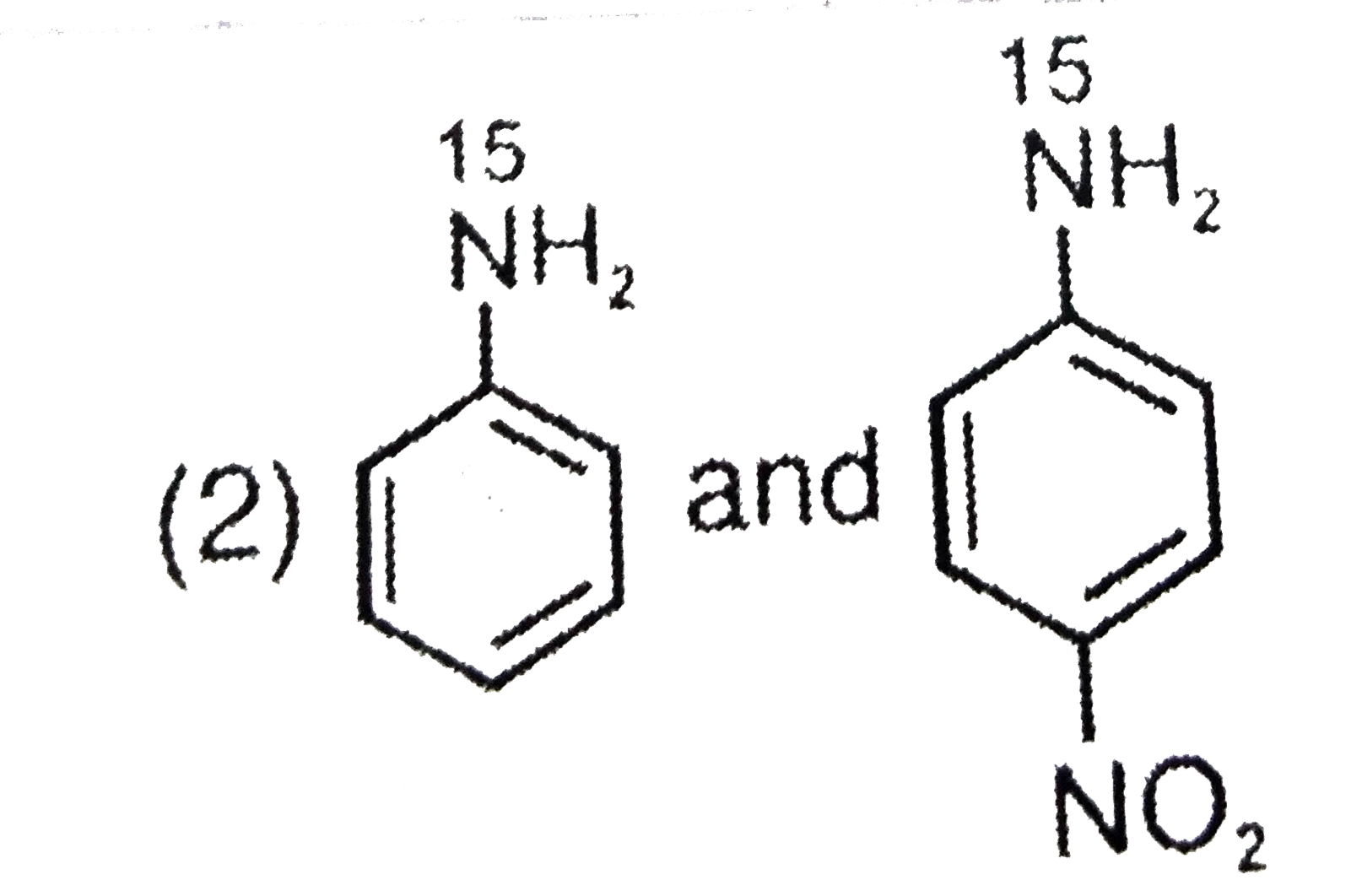
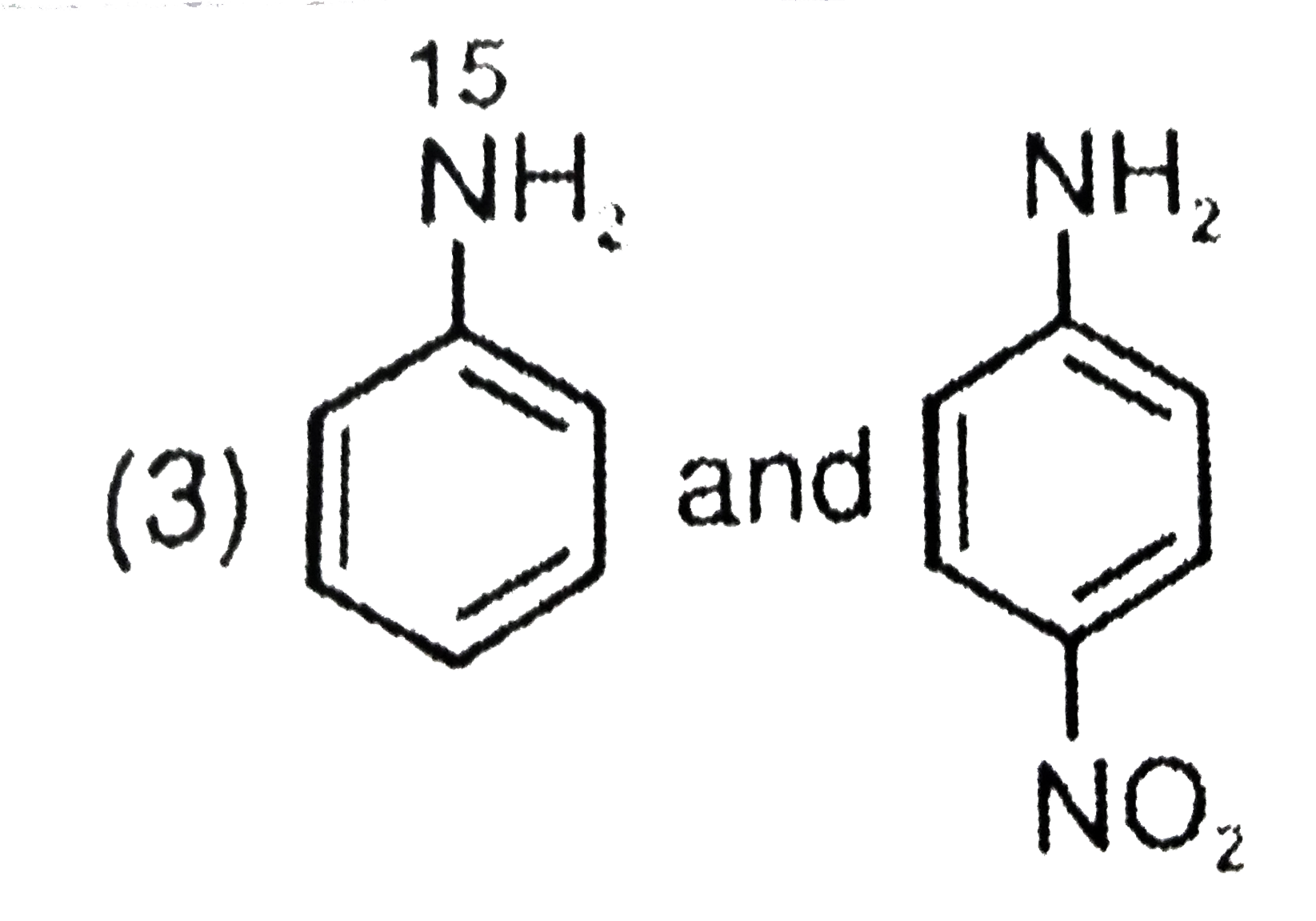
- The aqueous formed when a mixture of the following two amides (I and I...
Text Solution
|
- Which of the following does not give effervescence with NaHCO(3) ?
Text Solution
|
- The compound which does not give foul smell when heated with CHCl(3) &...
Text Solution
|
- The final product C, obtained in this reaction
Text Solution
|
- The action of bromine water (excess) on salicylic acid results in the ...
Text Solution
|
- 'S' is :
Text Solution
|
- Electrolytic reduction of nitrobenzene in weakly acidic medium gives .
Text Solution
|
- In the following reaction, X overset("Bromination")rarr Y underset(HCl...
Text Solution
|
- Predict the product
Text Solution
|
- Complete the following reaction
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p-toluidine form-
Text Solution
|
- An organic compound P on reduction gives compound Q which on reaction ...
Text Solution
|
- Primary amine reacts with carbon disulphide and HgCl(2) to produce alk...
Text Solution
|
- When aniline reacts with HNO(2)(NaNO(2)+HCl) diazonium chloride is for...
Text Solution
|
- When reacts with .............. To yield ..........as the final produc...
Text Solution
|
- Which of following statements is/are correct ?
Text Solution
|
- Which of following statement is/are correct ?
Text Solution
|
- Aniline can be obtained by reduction of nitrobenzene with
Text Solution
|