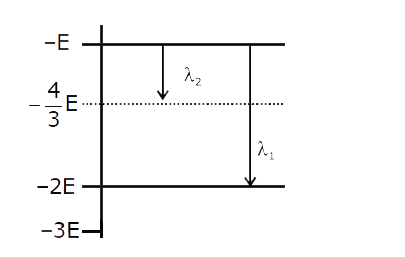
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective C (MCQs))|10 VideosATOMS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective D (MCQs))|3 VideosATOMS
MODERN PUBLICATION|Exercise COMPETITION FILE ( Objective B (MCQs))|20 VideosALTERNATING CURRENT
MODERN PUBLICATION|Exercise CHAPTER PRACTICE TEST|16 VideosCURRENT ELECTRICITY
MODERN PUBLICATION|Exercise Chapter Practice Test|15 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ATOMS -COMPETITION FILE (Objective BB (MCQs))
- The de-Broglie wavelength (lambda(B)) associated with the electron orb...
Text Solution
|
- If the series limit frequency of the Lyman series is v(L), then the se...
Text Solution
|
- An electron from various excited states of hydrogen atom emit radiatio...
Text Solution
|
- Radiation coming from transition n = 2 to n = 1 of hydrogen atoms fall...
Text Solution
|
- Taking the wavelength of first Balmer line in hydrogen spectrum (n = 3...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- If one were to apply Bohr model to a particle of mass 'm' and charge '...
Text Solution
|
- A hydrogen atom makes a transition from n=2 to n=1 and emits a photon....
Text Solution
|
- A neutron moving with a speed v makes a head-on collision with a hydro...
Text Solution
|
- The energy required to remove the electron from a singly ionized Hel...
Text Solution
|
- According to Bohr's theory, the time averaged magnetic field at the ce...
Text Solution
|
- The acceleration of electron in the first orbits of hydrogen atom is
Text Solution
|
- Some energy levels of a molecule are shown in the figure. The ratio of...
Text Solution
|
- An element of atomic number 9 emits K(alpha) X-ray of wavelength lamda...
Text Solution
|