Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise Level -3|5 VideosATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise Assessment Test 1|15 VideosATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise CONCEPT APPLICATION Level -1|39 VideosAIR AND OXYGEN
PEARSON IIT JEE FOUNDATION|Exercise Assemssment Test-2|15 VideosCHEMISTRY IN DAILY LIFE
PEARSON IIT JEE FOUNDATION|Exercise MOCK TEST|25 Videos
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-ATOMIC STRUCTURE AND TRANSFORMATION OF MATTER-Level -2
- The ratio of number of electrons present I four shells of an element i...
Text Solution
|
- The difference in the number of elelctrons between K, L and L, M shell...
Text Solution
|
- The colour change of CuSO(4) solution can observed when it is made to ...
Text Solution
|
- Identify the ratio of the coefficients of the products CuO,NO(2) and H...
Text Solution
|
- Which of the following chemical change is a photolytic decomposition r...
Text Solution
|
- While explaining modern concept of atom , Jack asked the teacher " wh...
Text Solution
|
- A stable neutral atom 'X' contains two completely filled orbits . Find...
Text Solution
|
- Mass of total positive charge present in an atom is 7348 times to th...
Text Solution
|
- While coming back from school in the school in the school bus , Bill a...
Text Solution
|
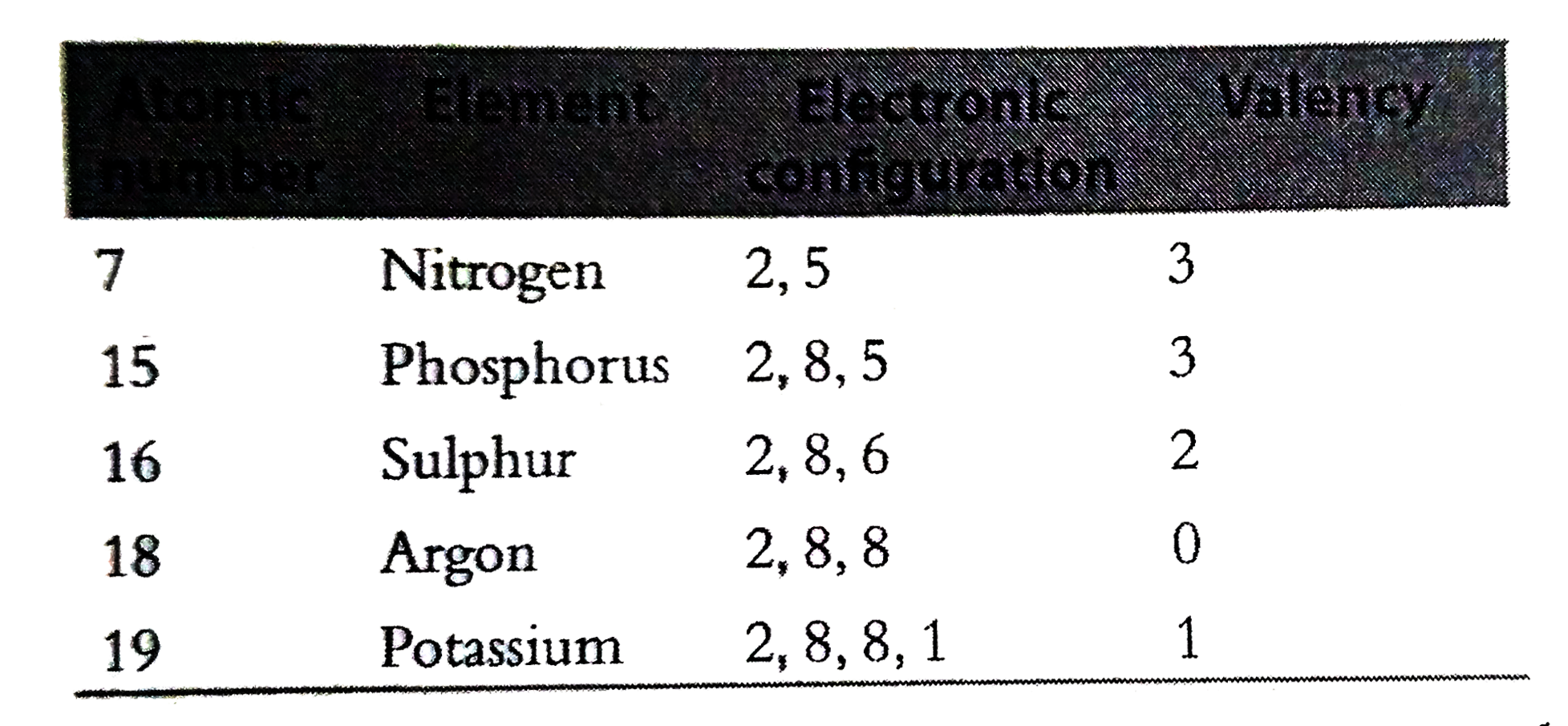
- Predict the valencies of the elements with atomic numbers 7 , 15 , 16 ...
Text Solution
|
- In an atom , L and M shells have the same number of electrons . N shel...
Text Solution
|
- Explain the formation of the compound formed by two elements with Z...
Text Solution
|
- An atom of an element has the electrons distributed in four shells ....
Text Solution
|
- A negative ion of an atom element X has 18 electrons and 16 protons ...
Text Solution
|
- If the formula of metal sulphite of a metal M is MSO(3) , give the fo...
Text Solution
|
- In a chemical laboratory , a student broke the thermometer while perf...
Text Solution
|
- Asha 's mother asked her daughter Asha to boil the milk and after some...
Text Solution
|
- What is the valency of iron in the products formed (a) When iron...
Text Solution
|
- Give the necessary chemical equations and balance them. (a) Reac...
Text Solution
|
- In a science fair , a student took two test tubes labelled A and B fi...
Text Solution
|