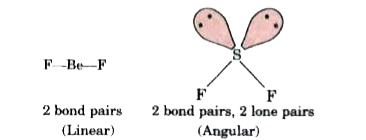Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (A. MULTIPLE CHOICE QUESTIONS)|35 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)|40 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(Long Answer Questions)|12 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-HIGHER ORDER THINKING SKILLS
- Explain why melting point of NaCl is higher than that of AlCl(3).
Text Solution
|
- Silver halides have law solubilities in water as compared to alkali me...
Text Solution
|
- Which homonuclear diatomic molecule besides O(2) is paramagnetic?
Text Solution
|
- Distinguish between antibonding and nonbonding orbitals.
Text Solution
|
- Anhydrous AlCl(3) is covalent but AlCl(3). 6H(2)O is ionic in nature. ...
Text Solution
|
- Cu^(+) and Na^(+) are of same size but CuCl is insoluble while NaCl is...
Text Solution
|
- The geometry of I(3)^(-) is
Text Solution
|
- The type of bonds present in NH(4)Cl are
Text Solution
|
- Which one has high boiling point and why? Ethyl alcohol or dimethyl ...
Text Solution
|
- XeF(2) molecule is linear molecule but it is sp^(3)d hybridized. Why?
Text Solution
|
- Bonds presents in CuSO(4) .5H(2)O is
Text Solution
|
- The following are aresome statement about oxides of VA group element ...
Text Solution
|
- The bond order in O2^-ion is
Text Solution
|
- Explain, why o-hydroxybenzaldehyde is a liquid at room temperature whi...
Text Solution
|
- A gaseous compound of nitrogen and oxygen is paramagnetic in nature. W...
Text Solution
|
- Draw three possible geometrical structures of PBr(2)Cl(3) and predict ...
Text Solution
|
- Bond angle in PH(4)^(+) is higher than that in PH(3).Why ?
Text Solution
|
- Why does PCl(5) exist as [PCl(4)]^(+)[PCl(6)]^(-) in the crystalline s...
Text Solution
|
- Explain the observations that the bond length in N^(+) is 0.02Å larger...
Text Solution
|
- IF molecular axis is X then which of the following overlapping will f...
Text Solution
|