A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS) (JEE (ADVANCED) FOR IIT ENTRANCE)|9 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|12 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)|40 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS) (JEE (MAIN) & OTHER STATE BOARDS FOR ENGINEERING ENTRANCE)
- Which one of the following properties is not shown by NO ? .
Text Solution
|
- The number of lone pairs of electrons on central atom of H(2)O, SnCl(2...
Text Solution
|
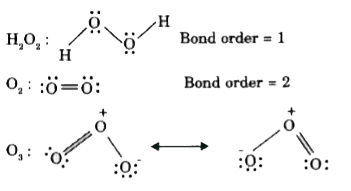
- The correct order of O - O bond length in O(2),H(2)O(2) and O(3) is
Text Solution
|
- Identify the T - shaped molecule in the following :
Text Solution
|
- The increassing order of bond order of O(2),O(2)^(+),O(2)^(-)and O(2)^...
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- The ground state magnetic property of B(2) and C(2) molecules will be
Text Solution
|
- Which of the following pairs have identical bond order?
Text Solution
|
- Which of the following species is not paramagnetic?
Text Solution
|
- What will be the shape of the compound MB(4)L(2)? Here B is bond pair ...
Text Solution
|
- What is the hybridisation and geometry of the given species? The speci...
Text Solution
|
- Which of the following has the strongest H - bond?
Text Solution
|
- The intramolecular hydrogen bond is present in
Text Solution
|
- According to molecular orbital theory, which of the following will not...
Text Solution
|
- Which of the following compounds contain(s) no covalent bond(s)? KCl...
Text Solution
|
- Total number of lone pair of electrons in 3 I(3)^(-) ion is
Text Solution
|
- According to molecular orbital theory, which of the following is true ...
Text Solution
|
- In which of the following processes, the bond order has increased and ...
Text Solution
|
- Two pi and half sigma bonds are present in :
Text Solution
|
- NO^(+) has bond order
Text Solution
|