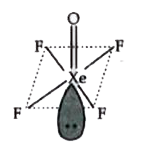
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|12 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS) (JEE (MAIN) & OTHER STATE BOARDS FOR ENGINEERING ENTRANCE)|53 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS) (JEE (ADVANCED) FOR IIT ENTRANCE)
- The molecular shapes of SF(4),CF(4) " and " XeF(4) are :
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Which of the following are isoelectronic and isostructural ? NO(3)^...
Text Solution
|
- Total number of lone pair of electrons in XeOF4 is :
Text Solution
|
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- The correct stability order of the following resonance structures is ...
Text Solution
|
- Assuming that Hund's rule is violated the bond order and magnetic natu...
Text Solution
|
- The species having pyramidal shape is
Text Solution
|
- The shapes of XeO(2)F(2) molecule is
Text Solution
|