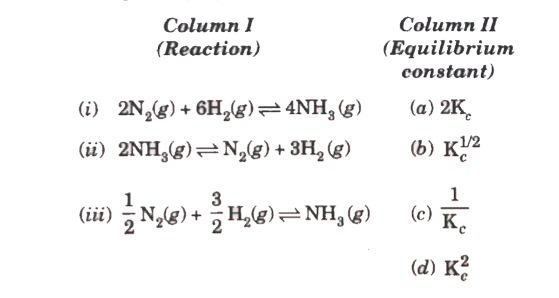
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
MODERN PUBLICATION|Exercise NCERT File ( Exemplar Problems Assertion and Reason)|7 VideosEQUILIBRIUM
MODERN PUBLICATION|Exercise NCERT File ( Exemplar Problems Long Answer Question )|4 VideosEQUILIBRIUM
MODERN PUBLICATION|Exercise NCERT File ( Exemplar Problems Short Answer Questions )|16 VideosENVIRONMENTAL POLLUTION
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|15 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|12 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-EQUILIBRIUM-NCERT File ( Exemplar Problems Matching )
- Match the following equilibria with the corresponding condition
Text Solution
|
- For the reaction N2(g) +3H2(g) hArr 2NH3(g) Equilibrium constant K...
Text Solution
|
- Match standard free energy of the reaction with the corresponding equi...
Text Solution
|
- Match the following species with the corresponding conjugate acid
Text Solution
|
- Match Column I with Column II .
Text Solution
|
- Match the following graphical variation with their description
Text Solution
|