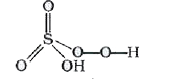
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - II )|35 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III )|2 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
MODERN PUBLICATION|Exercise Recent Examination Question|4 VideosSOLUTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-REDOX REACTIONS-Recent Examination Questions
- The oxidation state of S in Caro's acid (permono sulphuric acid) H(2)S...
Text Solution
|
- When sulphur dioxide is passed in an acidified K(2)Cr(2)O(7) solution ...
Text Solution
|
- Hydrogen gas is not liberated when the following metal added to dill H...
Text Solution
|
- In chromite are , the oxidation number of iron and chromium respective...
Text Solution
|
- In the reaction, 2FeSO(4)+H(2)SO(4)+H(2)O(2)toFe(2)(SO(4))(3)+2H(2)O. ...
Text Solution
|
- Pb(3)O(4)+4HNO(3)to2Pb(NO(3))(2)+PbO(2)+2H(2)O Which is true about th...
Text Solution
|
- Choose the correct statements applicable for the reaction : 2H(2)O+2...
Text Solution
|