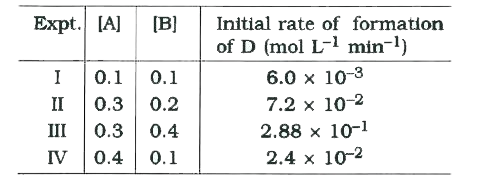A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
MODERN PUBLICATION|Exercise Multiple Choice Questions ( Effect of Temperature Catalyst and Radiation on Rate of Reaction )|13 VideosCHEMICAL KINETICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level-II)|50 VideosCHEMICAL KINETICS
MODERN PUBLICATION|Exercise Multiple Choice Questions ( Order and Molecularity of Reactions )|21 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Recent Examination Questions|17 VideosCHEMISTRY IN EVERY DAY LIFE
MODERN PUBLICATION|Exercise Recent Examination Questions|4 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS -Multiple Choice Questions ( Measuring Order of a Reaction : Integrated rate equation half life period )
- For the half life period of a first order reaction which one of the fo...
Text Solution
|
- The rate constant for a fist order reaction is k = 7xx10^(-4)s^(-1) .T...
Text Solution
|
- The following rate date were obtained at 313 K for the reaction : 2A...
Text Solution
|
- The half life period of a reaction is 100 min . In 400 min the intitia...
Text Solution
|
- A radioactive substance is reduced to 1/8 of its original concentratio...
Text Solution
|
- The rate for the first order reaction is 0.0069 mol L^(-1) min^(-1) an...
Text Solution
|
- The half life period of the reaction : N(2) O(5) rarr 2NO(2) +(1)/(2...
Text Solution
|
- If the initial concentration of reactants in a certain reaction is dou...
Text Solution
|
- The conversion of A to B follows second order kinetics. Doubling the c...
Text Solution
|
- For an nth order reaction the half life period t(1//2) is proportional...
Text Solution
|
- The rate of a first order reaction is 1.5xx10^(-2) mol L^(-1) "min"^(-...
Text Solution
|
- In a first order reaction ArarrB if k is rate constant and initial co...
Text Solution
|
- The half life of a reaction is inversely proportionaly to the square o...
Text Solution
|
- Half the period of a first order reaction is 1386 seconds . The specif...
Text Solution
|
- The rate law for reaction between the substances A and B is given by :...
Text Solution
|
- A reaction was found to be second order with respect to the concentrat...
Text Solution
|
- The rate law for the chemical reaction : 2NO(2)Clrarr 2NO(2)+Cl(2) ...
Text Solution
|
- For a zero order reaction linear plot was obtained for [A] vs t. The s...
Text Solution
|
- The reaction : X rarr Product follows first order kinetics . In 4...
Text Solution
|
- The rate constant for a first order reaction is 60 s^(-1) . The time r...
Text Solution
|