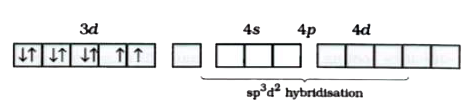
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
MODERN PUBLICATION|Exercise Multiple Choice Questions Level-III (Question from AIEEE/JEE Examinations)|11 VideosCOORDINATION COMPOUNDS
MODERN PUBLICATION|Exercise Recent Examination Questions|21 VideosCOORDINATION COMPOUNDS
MODERN PUBLICATION|Exercise Multiple Choice Questions Level-I : Basic Conceptual Qs (Theories of Bonding and Applications of Coordination Compounds)|22 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|7 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise Recent Examination Questions|17 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-COORDINATION COMPOUNDS-Multiple Choice Questions Level-II (Comprehensive Qs)
- When one mole of each of the following complex salts is treated with e...
Text Solution
|
- Which of the following will give a pair of enantiomorphs? (en = NH(2...
Text Solution
|
- What is crystal field splitting energy (CFSE)?
Text Solution
|
- The hybridization states of [Ni(CO)(4)], [Ni(CN)(4)]^(2-) and [NiCl(4)...
Text Solution
|
- Which of the following is an outer orbital complex ?
Text Solution
|
- Among the following which one is paramagnetic and has tetrahedral geom...
Text Solution
|
- Formula of hexaaquamanganese (II) phosphate is
Text Solution
|
- The complexes [Co(NH(3))(6)][Cr(CN)(6)] and [Cr(NH(3))(6)] [Co(CN)(6)]...
Text Solution
|
- The complex, [Pt(Py)(NH(3))BrCl] will have how many geometrical isomer...
Text Solution
|
- One mole of complex compound Co(NH(3))(5)Cl(3) gives 3 moles of ions o...
Text Solution
|
- Which one of the following complexes is outer orbital complex ?
Text Solution
|
- Which of the following cyano complexes would exhibit the lowest value ...
Text Solution
|
- The value of 'spin only' magnetic moment for one of the following conf...
Text Solution
|
- Which kind of isomerism is exhibited by octahedral Co(NH(3))(4)Br(2)Cl...
Text Solution
|
- The "spin only" magnetic moment of Ni^(2+) in aqueous solution would b...
Text Solution
|
- Which one of the following has a square planar geometry? (At. nos. ...
Text Solution
|
- The co-ordination number and the oxidation state of an element 'E' in...
Text Solution
|
- In which of the following octahedral complexes of Co(Z = 27) will the...
Text Solution
|
- Both [Ni(CO)(4)] and [Ni(CN)(4)]^(2-) are diamagnetic. The hybridisati...
Text Solution
|
- The IUPAC name of [Ni(NH(3))(4)] [NiCl(4)] is :
Text Solution
|