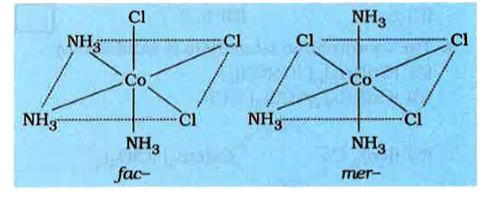
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
MODERN PUBLICATION|Exercise Recent Examination Questions|21 VideosCOORDINATION COMPOUNDS
MODERN PUBLICATION|Exercise Multiple Choice Questions Level-II (Comprehensive Qs)|45 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|7 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise Recent Examination Questions|17 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-COORDINATION COMPOUNDS-Multiple Choice Questions Level-III (Question from AIEEE/JEE Examinations)
- Which of the following pairs represents linkage isomers?
Text Solution
|
- A solution containing 2.675 g of CoCl(3).6NH(3) (molar mass = 267.5 g ...
Text Solution
|
- Which one of the following has an optical isomer ?
Text Solution
|
- The magnetic moment (spin only) of [NiCl(4)]^(2-) is :
Text Solution
|
- Which of the following facts about the complex [Cr(NH(3))(6)]Cl(3) is ...
Text Solution
|
- Among the ligands NH(3), en, CN^(-) and CO the correct order of their ...
Text Solution
|
- Which one of the following complex ions has geometrical isomers? (e...
Text Solution
|
- Which among the following will be named as dibromidobis (ethylene diam...
Text Solution
|
- Which of the following complex species is not expected to exhibit opti...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate li...
Text Solution
|
- The number of geometric isomers that can exist for square planar [Pt(C...
Text Solution
|