Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
MODERN PUBLICATION|Exercise NCERT FILE Solved (NCERT - Exemplar Problems) (Matching Type Questions)|2 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise NCERT FILE Solved (NCERT - Exemplar Problems) (Assertion and Reason Type Questions)|4 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise NCERT FILE Solved (NCERT - Exemplar Problems) (Multiple Choice Questions (Type - II)|5 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS ( based on the given. passage/comprehension )|8 VideosS-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-REDOX REACTIONS -NCERT FILE Solved (NCERT - Exemplar Problems) (Short Answer Questions)
- The reaction CI2(g) + 2OH^(-) (aq) rarrCIO^(-) (aq)+ CI^(-) (aq) + H2O...
Text Solution
|
- MnO4^(2-) undergoes disproportionation reaction in acidic medium but ...
Text Solution
|
- PbO and PbO(2) react with HCl according to following chemical equation...
Text Solution
|
- Nitric acid is an oxidising agent and reacts with PbO but it does not ...
Text Solution
|
- Write balanced chemical equations for the following reactions : (i) ...
Text Solution
|
- Calculate the oxidation number of phosphorus in the following species....
Text Solution
|
- Calculate the oxidation number of each sulphur atom in the following c...
Text Solution
|
- Balance the following equations by the oxidaiton number method . (i)...
Text Solution
|
- Identify the redox reaction out of the following reacitons and identif...
Text Solution
|
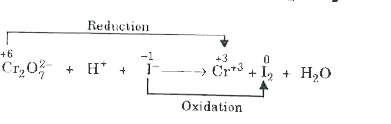
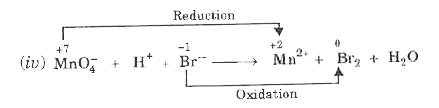
- Balance the following ionic equations (i) Cr2O7^(2-) +H^(+)I^(-) rar...
Text Solution
|