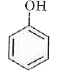
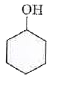
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE MULTIPLE CHOICE QUESTION (TYPE -II)|10 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE SHORT ANSWER TYPE QUESTIONS|28 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE NCERT (TEXTBOOK EXERCISES)|14 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -NCERT FILE NCERT (EXEMPLAR PROBLEMS) (MULTIPLE CHOICE QUESTION (TYPE -I))
- The source of nitrogen in Gabriel syntheisis of amine is..
Text Solution
|
- Amongst the given set of reactants, the most appropriate for preparing...
Text Solution
|
- The best reagent for converting 2-phenylpropanamide into 2-phenylpropa...
Text Solution
|
- The best reagent for converting, 2-phenylpropanamide into 1-phenyletha...
Text Solution
|
- Hoffmann bromamide degradation reaction is shown by
Text Solution
|
- The correct increasing order of basic strength for the following compo...
Text Solution
|
- Methylamine reacts with HNO(2) to form….
Text Solution
|
- The gas evolved when methylamine reacts with nitrous acid is….
Text Solution
|
- In the nitration of benzene using a mixture of conc. H(2)SO(4) and con...
Text Solution
|
- Reduction of aromatic nitro compounds using Fe and HCl gives
Text Solution
|
- The most reactive amine towards dilute hydrochloric acid is…
Text Solution
|
- Acid anhydrides on reaction with primary amine gives…
Text Solution
|
- The reaction ArN(2)^(+)Cl^(-) overset(Cu//HCl)to ArCl +N(2) + CuCl ...
Text Solution
|
- Best method for preparing primary amines from alkyl halides without ch...
Text Solution
|
- Which of the following compounds will not undergo azo coupling reactio...
Text Solution
|
- Which of the following compounds is the weakest Bronsted base?
Text Solution
|
- Among the following amines, the strongest Bronsted base is .
Text Solution
|
- The correct decreasing order of basic strength of the following specie...
Text Solution
|
- Which of the following should be most volatile? (I) CH(3)CH(2)CH(2)N...
Text Solution
|
- Which of the following methods of preparing of amines will give same n...
Text Solution
|