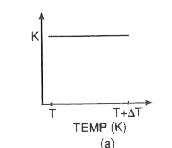
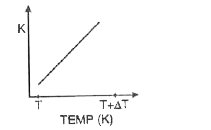
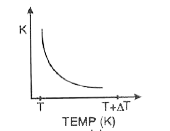
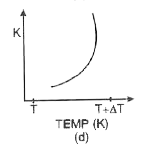
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT AND THERMODYNAMICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III) (Questions From AIEEE/JEE Examination)|17 VideosHEAT AND THERMODYNAMICS
MODERN PUBLICATION|Exercise Recent Competitve Questions|17 VideosHEAT AND THERMODYNAMICS
MODERN PUBLICATION|Exercise Recent Competitve Questions|17 VideosGRAVITATION
MODERN PUBLICATION|Exercise Recent Competitive Questions|8 VideosLAWS OF MOTION
MODERN PUBLICATION|Exercise Revision test|43 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HEAT AND THERMODYNAMICS-Multiple Choice Questions (LEVEL-II)
- Two blocks of ice join together where pressed. Which one of the follow...
Text Solution
|
- The equation of state for a real gas such as hydrogen oxygen etc. is c...
Text Solution
|
- An ideal gas is initiaily at temperature T and volumoeV. lts volume is...
Text Solution
|
- Which of the graphs shown in fig. correctly represents the variation o...
Text Solution
|
- For a monoatomic gas in adiabatic process, the relation between the pr...
Text Solution
|
- If the degrees of freedom of a gas are f, then the ratio of its specif...
Text Solution
|
- A volume V and temperature T was obtained, as shown in diagram, when a...
Text Solution
|
- A vessel containing air of mass 8g at 400 K is provided with a hole so...
Text Solution
|
- If one mole of a monoatomic gas gamma =(5)/(3), is mixed with one mole...
Text Solution
|
- Pure water cooled to -15^(@)C is contained in a thermally insulated fl...
Text Solution
|
- One mole of monoatomic gas is mixed with three moles of diatomic gas. ...
Text Solution
|
- Half a mole of helium is contained in a container at S.T.P. How much h...
Text Solution
|
- A Carnot's engine is working between 7^(@)C and 287^(@)C. It is desire...
Text Solution
|
- When a gas enclosed in a closed vessel was heated so as to increase it...
Text Solution
|
- One mole of an ideal gas at an initial temperature of TK does 6 R joul...
Text Solution
|
- The temperature of equal masses of three different liquids A, B and C ...
Text Solution
|
- A Carnot engine whose sink is at 300 K has an efficiency of 40%. By ho...
Text Solution
|
- Statement-I: The temperature at which centigrade and Fahrenheit thermo...
Text Solution
|
- Statement-I : It is not posible for a system, unaided by an external e...
Text Solution
|
- Statement-I : A beaker is completely filled with water at 4^(@)C. It w...
Text Solution
|