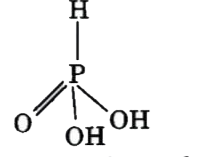
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY OF P - BLOCK ELEMENTS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III) (Question from AIEEE/JEE Examinations)|14 VideosCHEMISTRY OF P - BLOCK ELEMENTS
MODERN PUBLICATION|Exercise Recent Examination Questions|20 VideosCHEMISTRY OF P - BLOCK ELEMENTS
MODERN PUBLICATION|Exercise Recent Examination Questions|20 VideosCHEMISTRY OF D-AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise Recent Examination Questions|11 VideosCHEMISTRY OF s-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMISTRY OF P - BLOCK ELEMENTS-Multiple Choice Questions (Level - II) (Comprehensive Qs)
- Ozone gives brown colour with
Text Solution
|
- The number of S-S bonds in sulphur trioxide trimer (S(3)O(9)) is
Text Solution
|
- For H(3)PO(3) and H(3)PO(4) the correct choice is
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- Among the following the pair in which the two species are not iso - st...
Text Solution
|
- The correct order of acidic nature of oxides is in the order
Text Solution
|
- Na(2)S(2)O(3) is oxidised by I(2) to
Text Solution
|
- Which one statement about sulphur dioxide gas is incorrect?
Text Solution
|
- The correct order of increasing bond angles in the following species i...
Text Solution
|
- The paramagnetic oxides of nitrogen are
Text Solution
|
- The least stable hydride of 15th group elements is
Text Solution
|
- When Br(2) is treated with aqueous solutions of NaF, NaCl and NaI sepa...
Text Solution
|
- Which of the following statements is not valid for oxoacids of phospho...
Text Solution
|
- When Cl(2) reacts with hot and concentrated soldium, the oxidation num...
Text Solution
|
- Excess of PCl(5) reacts with conc. H(2)SO(4) giving
Text Solution
|
- The oxoacid of sulphur that contains a lone pair of electrons on sulph...
Text Solution
|
- The correct order of acidic stength is :
Text Solution
|
- The number of sigma - bonds in P4O(10) is :
Text Solution
|
- H(3)BO(3) is :
Text Solution
|
- Among Al(2)O(3), SiO(2),P(2)O(3) and SO(2), the correct order of acid ...
Text Solution
|