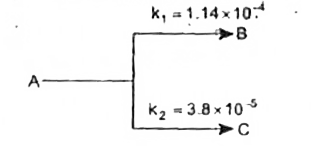
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Assignment problems (Objective) Level-II|21 VideosCHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Assignment problems (Objective) Level-II (Comprehension-I)|5 VideosCHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Assignment problems (Subjective) Level-II|15 VideosCHEMICAL EQUILIBRIUM
FIITJEE|Exercise MATCHING TYPE QUESTIONS|3 VideosCHEMISTRY IN EVERY DAY LIFE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-CHEMICAL KINETICS AND RADIOACTIVITY-Assignment problems (Objective) Level-I
- If a reaction with t(1//2)=69.3 sec, has a rate constant 10^(-2)sec^(-...
Text Solution
|
- The specific rate constant for a first order reaction is 60xx10^(-4) s...
Text Solution
|
- The rate constant for a zero order reaction is 2xx10^(-2) mol L^(-1)se...
Text Solution
|
- A first order reaction is carried out with an initial concentration of...
Text Solution
|
- What fraction of a rectant showing first order remains after 40min, if...
Text Solution
|
- A substance undergoes a first order decomposition. The decomposition f...
Text Solution
|
- A tangent drawn on the curve obtained by plotting concentration of pro...
Text Solution
|
- For reaction , 3A to products, it is found that the rate of reaction ...
Text Solution
|
- The thermal decomposition of acetaldehyde , CH(3)CHO to CH(4)+CO, has ...
Text Solution
|
- The reaction , 2O(3) to 3O(2) , is assigned the following mechanism. ...
Text Solution
|
- For an endothermic reaction where DeltaH represents the enthalpy of th...
Text Solution
|
- The rate expression for a reaction is rate =k[A]^(3//2)[B]^(-1), the o...
Text Solution
|
- The reaction, A(g)+2B(g) to C(g)+D(g) is an elementary process. In an ...
Text Solution
|
- If concentration are measured in moles/lit and time in minutes, the un...
Text Solution
|
- A radioactive element has a half-life period of 140 days . How much of...
Text Solution
|
- For a first order reaction, the plot of log[A](t)vs t is linear with a
Text Solution
|
- For a hypothetical reaction, A+B to C+D, the rate =k[A]^(-1//2)[B]^(3/...
Text Solution
|
- In a reaction the threshold energy is equal to
Text Solution
|
- For a first reaction, t(0.75) is 138.6 seconds. Its specific rate cons...
Text Solution
|
- At the given point of intersection of the two curves shown concentrati...
Text Solution
|