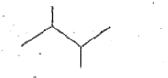
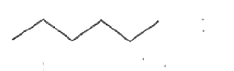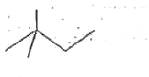A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - A) (Obejctive type question)|55 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - B) (Obejctive type question)|13 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise TRY YOURSELF|78 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-Exercise
- Identify A
Text Solution
|
- Which of the following reaction cannot be used for the preparation of ...
Text Solution
|
- Which of the following has maximum boiling point?
Text Solution
|
- Which of the following halogens is the most reactive?
Text Solution
|
- Which of the following alkane upon dichlorination can give only two pr...
Text Solution
|
- Which of the following has maximum angle strain ?
Text Solution
|
- Total number of conformation of ethane is :
Text Solution
|
- Conformations arise due to rotation around
Text Solution
|
- Which of the following is the most stable cycloalkane ?
Text Solution
|
- Bond angle in chair form of cyclohexane is
Text Solution
|
- Most stable conformation of n-butane is :
Text Solution
|
- Torsion strain is the repulsive interaction between
Text Solution
|
- Which form (s) of cyclohexane is/are free from angle strain?
Text Solution
|
- The number of axial hydrogen atoms in chair form of cyclohexane is
Text Solution
|
- Select the correct statement
Text Solution
|
- In which of the following geometrical isomerism is possible ?
Text Solution
|
- Identify the product in the following reaction
Text Solution
|
- Which of the following is not a possible product in the above reaction...
Text Solution
|
- Consider the given reaction CH(3)-underset(CH(3))underset(|)overset(...
Text Solution
|
- Identify X
Text Solution
|