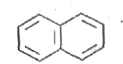
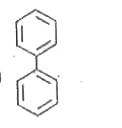
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - B) (Obejctive type question)|13 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - C) (Previous Years Questions)|69 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Exercise|50 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-Assignment(Section - A) (Obejctive type question)
- Which one is most stable ?
Text Solution
|
- The chair form is stable than boat form by potential energy kJ/mol
Text Solution
|
- Which one is not aromatic compound ?
Text Solution
|
- The incorrect match is
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- The possible compound A is
Text Solution
|
- On electrolysis of sodium succinate, the alkene obtained is and natur...
Text Solution
|
- Which one is not prepared by Wurtz reaction ?
Text Solution
|
- can be prepared by
Text Solution
|
- Mg(2)C(3) + H(2)O to A overset(Na)to B overset(CH(3)Br)to C The inco...
Text Solution
|
- In the following compunds the decreasing order of B.P. is (i) CH(3)-...
Text Solution
|
- CH(3)-overset(CH(3))overset(|)CH-CH(2)-CH(3) overset(Cl(2))underset("U...
Text Solution
|
- The compound A is
Text Solution
|
- In Iodination for preparation of iodomethane compound used is
Text Solution
|
- In chlorination the relation rate of abstraction of H in 3^(@), 2^(@) ...
Text Solution
|
- In which alkane isomerization will not occur ?
Text Solution
|
- Compound A is :
Text Solution
|
- The minimum heat of hydrogenation is in
Text Solution
|
- The compound A is
Text Solution
|
- In which of the following reactions Markovnikov's rule is not observed...
Text Solution
|