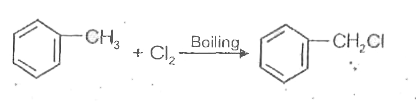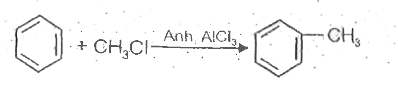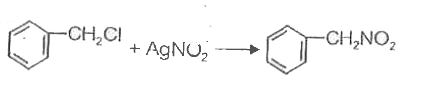A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - B) (Obejctive type question)|13 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-Assignment(Section - C) (Previous Years Questions)
- Which of the compounds with molecular formula C(5)H(10)yields acetone ...
Text Solution
|
- Anti-Markownikoff's addition of HBr is not observed in
Text Solution
|
- Given I and II are
Text Solution
|
- Which of the following conformers for ethylene glycol is most stable?
Text Solution
|
- Reaction of HBr with propene in the presence of peroxide gives :-
Text Solution
|
- Which one of the following is a free-radical substituion reaction ?
Text Solution
|
- Using anhydrous AlCl(3) as catalyst, which one of the following reacti...
Text Solution
|
- Which of the following compounds undergoes mucleophilic substitution r...
Text Solution
|
- Condiser the reactions, (i) (CH(3))(2)CH-CH(2)Broverset(C(2)H(5)OH)r...
Text Solution
|
- Which will undergo fastest S(N)2 substitution reaction when treated wi...
Text Solution
|
- Y in the reaction is
Text Solution
|
- The number of isomers that can be obtained theoretically on monochlori...
Text Solution
|
- When CH(3)CH(2)CHCl(2) is treated with NaNH(2) the product formed is
Text Solution
|
- 2-bromopentane is heated with postassium ethoxide in ethano1 The major...
Text Solution
|
- An organic compound A(C(4)H(6)CI) on reation withNa/diethyl ether giv...
Text Solution
|
- In the following reaction , C(6)H(5)CH(2)Broverset(1.Mg,Ether)underset...
Text Solution
|
- When 3,3-dimethyl-2-butanol is heated with H(2)SO(4) the major product...
Text Solution
|
- Identify Z in the sequence of reaaction CH(3)-CH(2)-CH=CH(2) overset...
Text Solution
|
- In the following reaction The major product is
Text Solution
|
- What products are formed when the following compound is treated with B...
Text Solution
|