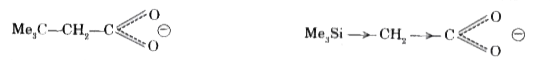Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise NCERT FILE (NCERT IN TEXT QUESTIONS )|8 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise NCERT FILE (NCERT TEXTBOOK EXERCISES )|20 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise Conceptula Questions 1|14 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST (FOR BOARD EXAMINATION)|12 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST FOR BOARD EXAMINATION|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS-Conceptula Questions 2
- Aromatic carboxylic acids do not undergo Friedel crafts reaction...
Text Solution
|
- pKa value of 4- nitrobenzoic acid to lower than that of benzoic...
Text Solution
|
- Write the IUPAC name of (I ) HOOC -COOH " " (ii ) COOH -CH2 ...
Text Solution
|
- Why are the boiling points of carboxylic acids higher than the corres...
Text Solution
|
- Carboxylic acids do not give the characteristic reactions of carbonyl ...
Text Solution
|
- Formic acid reduces Tollen's reagent because
Text Solution
|
- (CH(3))(3)C CH(2)COOH is more acidic than (CH(3))(3)SiCH(2)COOH becaus...
Text Solution
|
- Give reasons for the following in one or two sentences. 'Acetic acid...
Text Solution
|
- Arrange the following in the decresing order of acidic strength ...
Text Solution
|
- Why is the bond length of C=O in carboxylic acids slightly la...
Text Solution
|
- What is glacial acetic acid ? Why is it so named ?
Text Solution
|
- Complete the following :
Text Solution
|
- Arrange the following compounds in the increasing of their acid...
Text Solution
|
- Highly branched carboxylic acids are less acidie than unbranche...
Text Solution
|
- Arrange the following compounds in increasing order of their acid...
Text Solution
|