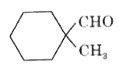
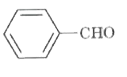
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise NCERT FILE (NCERT EXEMPLAR PROBLEMS ) (MULTIPLE CHOICE QUESTIONS ( TYPE -II)|6 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise NCERT FILE (NCERT EXEMPLAR PROBLEMS ) (SHORT ANSWER TYPE QUESTIONS )|19 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise NCERT FILE (NCERT TEXTBOOK EXERCISES )|20 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST (FOR BOARD EXAMINATION)|12 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST FOR BOARD EXAMINATION|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS-NCERT FILE (NCERT EXEMPLAR PROBLEMS ) (MULTIPLE CHOICE QUESTIONS ( TYPE -I)
- Addition of water to alkynes occurs in acidic medium and in the presen...
Text Solution
|
- Which of the following compounds is most reactive towards nucleophilic...
Text Solution
|
- The correct order of increasing acidic strength is .
Text Solution
|
- Compound Ph-O-overset(O)overset(||)(C)-Ph can be prepared by the react...
Text Solution
|
- The reagent which does not react with both acetone and benzaldehyde is
Text Solution
|
- Cannizaro's reaction is not given by
Text Solution
|
- Which product is formed when the compound concentrated aqueous K...
Text Solution
|
- CH3 -C-= CH underset(1% Hg SO4 ) overset(40% H2SO4 )to A overset(...
Text Solution
|
- Compounds (A) and (C ) in the following reactions are CH(3)CHO overs...
Text Solution
|
- Which is the most suitable reagent for the following conversion ...
Text Solution
|
- Which of the following compound will give butanone on oxidation with a...
Text Solution
|
- In Clemmensen Reduction carbonyl compound is treated with
Text Solution
|