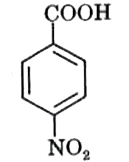
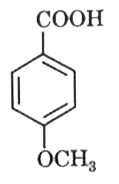
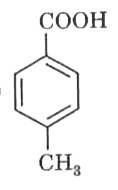
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - PASSAGE BASED QUESTIONS )|10 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - ASSERTION REASON QUESTIONS )|10 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise QUICK MEMORY TEST ( C. CHOOSE THE CORRECT ALTERNATIVE )|19 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST (FOR BOARD EXAMINATION)|12 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST FOR BOARD EXAMINATION|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS-REVISION EXERCISES (OBJECTIVE QUESTIONS - MULTIPLE CHOICE QUESTIONS )
- Which on heating with aqueous KOH, produces acetaldehyde?
Text Solution
|
- Iodoform test is not given by
Text Solution
|
- In the following, strongest acid is :
Text Solution
|
- in the following reaction , product P is
Text Solution
|
- Acetaldehyde and acetone can be distinguished by
Text Solution
|
- A carbonyl group can be converte into-CH(2) group by:
Text Solution
|
- Among the following compounds, which will not repond to cannizzaro's r...
Text Solution
|
- The IUPAC name of PhCH2 CH2 COOH is named as
Text Solution
|
- Which of the following compound undergoes haloform reaction?
Text Solution
|
- Which of the following is most acidic ?
Text Solution
|
- Which of the following is the strongest acid:
Text Solution
|
- What type of organic compounds are prepared by Gattermann-Koch reactio...
Text Solution
|
- the IUPAC name of the compound is
Text Solution
|
- In the reaction the product P is
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|
- Match the name reaction ( column I) with the reagent ( column II...
Text Solution
|