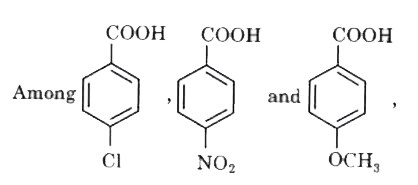
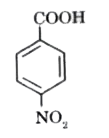
Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - ASSERTION REASON QUESTIONS )|10 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - VERY SHORT ANSWER QUESTIONS )|39 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - MULTIPLE CHOICE QUESTIONS )|45 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST (FOR BOARD EXAMINATION)|12 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST FOR BOARD EXAMINATION|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS-REVISION EXERCISES (OBJECTIVE QUESTIONS - PASSAGE BASED QUESTIONS )
- Aldehydes and ketones undergo nucleophilic addition reactions. With we...
Text Solution
|
- Aldehydes and ketones undergo nucleophilic addition reactions. With we...
Text Solution
|
- Aldehydes and ketones undergo nucleophilic addition reactions. With we...
Text Solution
|
- Aldehydes and ketones undergo nucleophilic addition reactions. With we...
Text Solution
|
- Aldehydes and ketones undergo nucleophilic addition reactions. With we...
Text Solution
|
- Carboxylic acids are distinctly acidic and ionise in water to give H3 ...
Text Solution
|
- Carboxylic acids are distinctly acidic and ionise in water to give H3 ...
Text Solution
|
- Carboxylic acids are distinctly acidic and ionise in water to give H3 ...
Text Solution
|
- Carboxylic acids are distinctly acidic and ionise in water to give H3 ...
Text Solution
|
- Carboxylic acids are distinctly acidic and ionise in water to give H3 ...
Text Solution
|