A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY-Exercise
- A silver coin weighing 11.34 g was dissolved in nitric acid When sodiu...
Text Solution
|
- 25 mL of a solution of barium hydroxide on titration with a 0.1 molar ...
Text Solution
|
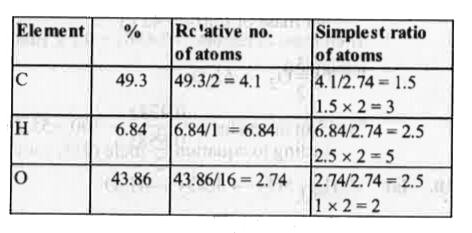
- An organic compound contains 49.3 % carbon,6.84 % hydrogen and its vap...
Text Solution
|
- The masses of carbon, hydrogen and oxygen in an organic compound are ...
Text Solution
|
- A gaseous hydrocarbon gives upon combustion, 0.72 g of water and 3.08 ...
Text Solution
|
- The chloride of a metal (M) contains 65.5% of chlorine. 100 ml of the ...
Text Solution
|
- In the reaction, 4NH(3)(g)+5O(2)(g) rarr 4NO(g)+6H(2)O(g), when 1 mole...
Text Solution
|
- If potassium chlorate is 80% pure, then 48 g of oxygen would be produc...
Text Solution
|
- When burnt in air, 14.0 g mixture of carbon and sulphur gives a mixtu...
Text Solution
|
- Consider the reaction 2 A+B+3C to P+2Q. Starting with 3 mol of A, 2 m...
Text Solution
|
- A mixture of CO and CO(2) having a volume of 20 mL is mixed with X mL...
Text Solution
|
- Consider a titration of potassium dichromate solution with acidified M...
Text Solution
|
- On dividing 0*25 by 22*1176, the actual answer is 0*011303. The correc...
Text Solution
|
- The number of significant figures for the three numbers 161 cm, 0.161...
Text Solution
|
- In compound A, 1.00 g nitrogen units with 0.57 g oxygen. In compound B...
Text Solution
|
- In the final answer of the expression ((29.2-20.2)(1.79xx10^(5)))/1.37...
Text Solution
|
- Specific volume of cylindrical virus particle is 6.02xx10^(-2) c c//g ...
Text Solution
|
- The maximum number of molecules is present in
Text Solution
|
- If N(A) is Avogadro's number then number of valence electrons in 4.2 g...
Text Solution
|
- 10 g of a metal gives 14 g of its oxide. The equivalent mass of its o...
Text Solution
|