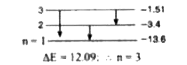
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-STRUCTURE OF ATOM-Exercise
- A beam of specific kind of particles of velocity 2.1 xx 10^7 m//s is s...
Text Solution
|
- An electron in a hydrogen atom in its ground state absorbs 1.5 times a...
Text Solution
|
- H-atom is exposed to electromagnetic radiation of lambda=1025.6 Å and ...
Text Solution
|
- If the lowest energy X-rays have lambda=3.055xx10^(-8) m, estimate the...
Text Solution
|
- Balmer gave an equation for wavelegth of visible region of H-spectrum ...
Text Solution
|
- In hydrogen atomic spectrum , a series limit is found at 12186.3 cm^(...
Text Solution
|
- If the average life time of an excited state of hydrogen is of the ord...
Text Solution
|
- Consider the following radial distribution function diagrams. Which of...
Text Solution
|
- Which statement (s) are correct ? (1) A 3s sub - shell has a maxim...
Text Solution
|
- In a multi - electron atom , which of the following orbitals descri...
Text Solution
|
- The numbers of radial nodes of 3s and 2p orbitals are respectively:
Text Solution
|
- The electronic, identified by quantum numbers n and l, (i) n = 4, l = ...
Text Solution
|
- Which of the following radiation distribation graph coreesponds to ...
Text Solution
|
- If n = 6, the correct sequence for filling of electrons will be.
Text Solution
|
- An electron is allowed to move freely in a closed cubic box of length ...
Text Solution
|
- Ground state energy of H-atom is (-E(1)),t he velocity of photoelectro...
Text Solution
|
- The dissociation energy of H(2) is 430.53 kJ mol^(-1), If H(2) is of d...
Text Solution
|
- The energy of a I,II and III energy levels of a certain atom are E,(4E...
Text Solution
|
- A 600 W mercury lamp emits monochromatic radiation of wavelength 331.3...
Text Solution
|
- The uncertainty in the position of an electron (mass = 9.1 xx 10^-28 g...
Text Solution
|