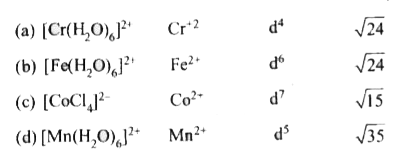A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- Which of the following is an example of homoleptic complex ?
Text Solution
|
- Which one of the following complexes shows optical isomerism ? (en=e...
Text Solution
|
- The pair having the same magnetic moment is [at. No. Cr = 24, Mn = ...
Text Solution
|
- Which molecule/ion among the following cannot act as a ligand in compl...
Text Solution
|
- The correct statement on the isomerism associated with the follow...
Text Solution
|
- The number of geometric isomers that can exist for square planner comp...
Text Solution
|
- An octahedral complex of Co^(3+) is diamagnetic . The hydridisation in...
Text Solution
|
- The correct statement about the magnetic properties of [Fe(CN)(6)]^(3-...
Text Solution
|
- An octahedral complex with molecular composition M.5 NH3 , Cl. SO4 has...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate lig...
Text Solution
|
- The correct IUPAC name for [Pt(C(5)H(5)N)(4)][PtCI(4)] complex
Text Solution
|
- [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2 are related to each other as
Text Solution
|
- The pair of compounds having metals in their highest oxidation state i...
Text Solution
|
- The number of geometric isomers that can exist for square planner comp...
Text Solution
|
- The geometries of Ni(CO)(4) and Ni(PPh(3))(2)Cl(2) are .
Text Solution
|
- [Co(NH3)5NO2]Cl2 and [Co(NH3)5(ONO)]Cl2 are related to each other as
Text Solution
|
- [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2 are related to each other as
Text Solution
|
- Which of the following complex does not show geometrical isomerism ?
Text Solution
|
- Which statement about coordination number of a cation is true?
Text Solution
|
- Select the correct code about complex [Cr(NO(2))(NH(3))(5)][ZnCl(4)] :...
Text Solution
|