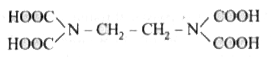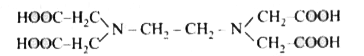A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- Which of the following compounds shows optical isomerism?
Text Solution
|
- The ionisation isomer of [Cr(H(2)O)(4) Cl (NO(2))]Cl
Text Solution
|
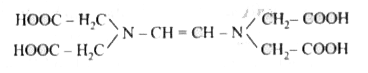
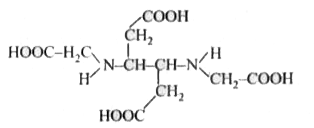
- The correct structure of ethylenediamineteraacetic acid (EDTA) is .
Text Solution
|
- Which one of the following complex is not expected to exhibit isomeris...
Text Solution
|
- The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the ex...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- The sum of coordination number and oxidation number of the metal M in ...
Text Solution
|
- Which of the following is the most likely structure of CrCl3*6H2O, if ...
Text Solution
|
- Which of the following is not chelating agent (a) Thiosulphate (b)...
Text Solution
|
- Which of the following species is not expected to be a ligand?
Text Solution
|
- Which is the pair of ambidentate ligand?
Text Solution
|
- Number of water molecules acting as ligands in CuSO(4).5H(2)O,ZnSO(4...
Text Solution
|
- Which of the following pair of complexes have the same EAN of the cent...
Text Solution
|
- The correct name of
Text Solution
|
- In octaamine -mu-dihydroxodiiron(III)sulphate, the number of bridging ...
Text Solution
|
- Ammonia will not form complex with
Text Solution
|
- Which of the following complex compound is low spin, inner orbital , d...
Text Solution
|
- An aqueous solution of titanium bromide shows zero magnetic moment. As...
Text Solution
|
- Which of the following complexes have a maximum number of unpaired ele...
Text Solution
|
- The degeneracy of d-orbitals is lost under: (I) Strong field ligand ...
Text Solution
|