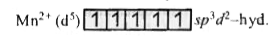A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- The degeneracy of d-orbitals is lost under: (I) Strong field ligand ...
Text Solution
|
- Relative to the average energy in the spherical crystal field the t(2g...
Text Solution
|
- Which of the following outer orbital complex has the highest magnetic ...
Text Solution
|
- the correct IUPAC name of the following compound [Cr(NH3)5(NCS)][ZnCl4...
Text Solution
|
- Mn^(2+) forms a complex with Br– ion. The magnetic moment of the compl...
Text Solution
|
- Which of the following hydrate is diamagnetic ?
Text Solution
|
- Which one of the following will show paramagnetism corresponding to 2 ...
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|
- [Se(H2 O)6]^(3+) ion is
Text Solution
|
- The crystal field stabilization energy (CFSE) is the highest for
Text Solution
|
- Which of the following complex ion is not expected to absorb visible l...
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- A solution containing 2.675 g of CoCl(3).6NH(3) (molar mass = 267.5 g ...
Text Solution
|
- Of the following complex ions, which is diamagnetic in natures?
Text Solution
|
- The d-electron configurations of Cr^(2+), Mn^(2+), Fe^(2+) and Co^(2+)...
Text Solution
|
- Which of the following complex compounds will exhibit highest paramagn...
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Red precipitae is obtained when ethanol solution of dimethylglyoxime i...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|