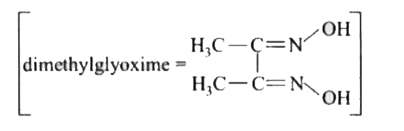A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- Which of the following complex compounds will exhibit highest paramagn...
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Red precipitae is obtained when ethanol solution of dimethylglyoxime i...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|
- Which of the following complexes is used as an anti-cancer agent:
Text Solution
|
- Cobalt (III) chloride forms several octahedral complexes with amonia. ...
Text Solution
|
- HgCl(2) and I(2) both when dissolved in water containing I^(-) ions th...
Text Solution
|
- Correct increasing order for the wavelength of absorption in the visib...
Text Solution
|
- Pick out the correct statement with respect to [Fe(CN)6]^(3-)
Text Solution
|
- The molar ionic conductances of octahedral complexes. (I) PtCl(4).5N...
Text Solution
|
- Which of the following has the highest molar conductivity in solution?
Text Solution
|
- Consider the following complex [Co(NH(3))(5)CO(3)]ClO(4) The coordin...
Text Solution
|
- Nickel (Z=28) combines with a uninegative monodenatate ligands to for...
Text Solution
|
- Ferrocene is
Text Solution
|
- An example of organometallic compound is
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following is an organometallic compound?
Text Solution
|
- In Fe(CO)5, the Fe-C bond possesses:
Text Solution
|
- Which of the following is not considered as an organometallic compound...
Text Solution
|