A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- Which of the following has the highest molar conductivity in solution?
Text Solution
|
- Consider the following complex [Co(NH(3))(5)CO(3)]ClO(4) The coordin...
Text Solution
|
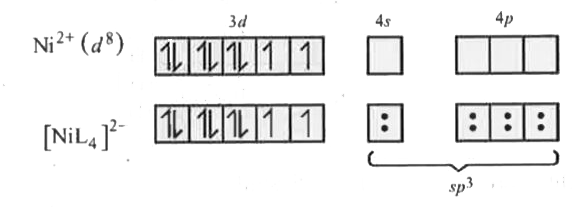
- Nickel (Z=28) combines with a uninegative monodenatate ligands to for...
Text Solution
|
- Ferrocene is
Text Solution
|
- An example of organometallic compound is
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following is an organometallic compound?
Text Solution
|
- In Fe(CO)5, the Fe-C bond possesses:
Text Solution
|
- Which of the following is not considered as an organometallic compound...
Text Solution
|
- CH(3)MgBr is an organometallic compound due to
Text Solution
|
- Oxidation state of "V" in Rb(4)K[HV(10)O(28)] is .
Text Solution
|
- Following Sidgwick's rule of EAN, Co(CO)(x) will be.
Text Solution
|
- Coordination compounds have great importance in biological systems. In...
Text Solution
|
- Carbonyls are organometallic compounds .
Text Solution
|
- Which of the following carbonyls will have the strongest C-O bond
Text Solution
|
- Which of the following has longest C-O bond length? (Free C-O bond len...
Text Solution
|
- An example of a sigma bonded organometallic compound is:
Text Solution
|
- Facial-meridional isomers is associated with which one of the followin...
Text Solution
|
- The IUPAC name of the red coloured complex [Fe(C(4)H(7)O(2)N(2))(2)] o...
Text Solution
|
- The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the ex...
Text Solution
|