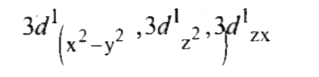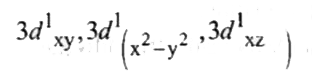A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-COORDINATION COMPOUNDS -Exercise
- The hypothetical complex chloro diaquatriammine cobalt (II) chloride c...
Text Solution
|
- 50 ml of 0.2 M solution of a compound with empirical formula CoCl(3)....
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- Arrange the following in increasing value of magnetic moments. (i) ...
Text Solution
|
- Which of the following complex is an outer orbital complex?
Text Solution
|
- Which of the following order of stability of complex ion is Incorrect ...
Text Solution
|
- The correct order of the stoichiometries of AgCl formed when AgNO3 in ...
Text Solution
|
- Which of the following statement is correct for the complex Ca(2)[Fe(C...
Text Solution
|
- The magnetic moment of complex (A) of Co was found to be 4.89BM and th...
Text Solution
|
- In which of the following species the hybrid state of the central atom...
Text Solution
|
- Which of the following molecule do not have the same number of unpaire...
Text Solution
|
- Which of the following complex ion(s) is/are not expected to absorb vi...
Text Solution
|
- The d-electron configurations of Cr^(2+), Mn^(2+), Fe^(2+) and Co^(2+)...
Text Solution
|
- The oxidation number of Co in the complex ion
Text Solution
|
- Match the geometry (given in columnA) with the complexes (given in col...
Text Solution
|
- [Fe(en)(2)(H(2)O)(2)]^(2+) +en to " complex(X)". The correct statement...
Text Solution
|
- [NiCl(2){P(C(2)H(5))(2)(C(6)H(5))}(2)] exhibits temperature dependent ...
Text Solution
|
- Which of the following organometallic compound is a sigma and pi bonde...
Text Solution
|
- Among the following, which is not the pi-bonded organometallic compoun...
Text Solution
|
- The coordination number, EAN of the central metal atom and geometry of...
Text Solution
|