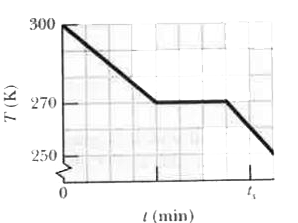
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT-MEASUREMENT AND TRANSFER
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS|60 VideosHEAT-MEASUREMENT AND TRANSFER
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS(MORE THAN ONE CORRECT CHOICE TYPE )|8 VideosHEAT-MEASUREMENT AND TRANSFER
RESNICK AND HALLIDAY|Exercise CHECKPOINT 2|1 VideosGRAVITATION
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (INTEGER TYPE)|4 VideosHYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS(Integer Type)|6 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-HEAT-MEASUREMENT AND TRANSFER-PROBLEMS
- A sphere of radius 0.350m, temperature 27.0^(@)C , and emissivity 0.85...
Text Solution
|
- If you were to walk brieftly in space without a spacesuit while far fr...
Text Solution
|
- Ice has formed on a shallow pond, and a steady state has been reached,...
Text Solution
|
- Penguin huddling.To withstand the harsh weather of the Antartic, emper...
Text Solution
|
- The giant hornet Vespa mandarinia japonica preys on Japanes bees.Howev...
Text Solution
|
- Calculate the minimum amount of energy, in joules, required to complet...
Text Solution
|
- What mass of butter, which has a usable energy content of 6.0 KCal //g...
Text Solution
|
- (a) Two 40g ice cubes are droped into 200g of water in a thermally ins...
Text Solution
|
- A 0.600kg sample is placed in a cooling apparatus that removes energy ...
Text Solution
|
- A cylindrical copper rod of length 0.60m and cross-sectional area 6.0 ...
Text Solution
|
- In a solar water heater, energy from the Sun is gathered by water that...
Text Solution
|
- A person makes a quantity of iced tea by mixing 250g of hot tea (essen...
Text Solution
|
- A 150 g copper bowl contains 260 g of water, both at 20.0^(@)C A very ...
Text Solution
|
- The temperature of a body falls from 40^(@)C to 36^(@)C in 5 minutes w...
Text Solution
|
- The earth receives solar radiation at a rate of 8.2Jcm^(-2)min^(-1) . ...
Text Solution
|
- A hot body placed in air is cooled down according to Newton's law of c...
Text Solution
|
- (a) Calculate the rate at which body heat is conducted through the clo...
Text Solution
|
- Find the rate of heat flow through a cross section of the rod shown in...
Text Solution
|
- A calorimeter containes 50g of water at 50^(@)C . The temperature fall...
Text Solution
|
- A metal ball of mass 1kg is heated by means of a 20W heater in a room ...
Text Solution
|