Text Solution
Verified by Experts
Topper's Solved these Questions
THE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( SINGLE CORRECT CHOICE TYPE )|48 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( MORE THAN ONE CORRECT CHOICE TYPE )|9 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise CHECK POINT|8 VideosTEMPERATURE, ZEROTH LAW OF THERMODYNAMICS AND THERMAL EXPANSION
RESNICK AND HALLIDAY|Exercise PRACTICE QUETIONS (Integer Type)|4 VideosTHE KINETIC THEORY OF GASES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS|72 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-THE FIRST LAW OF THERMODYNAMICS-PROBLEMS
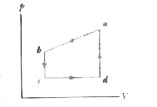
- A gas within a closed chamber undergoes the cycle shown in the p-V dia...
Text Solution
|
- As a gas is held within a closed chamber, it passes through the cycle ...
Text Solution
|
- Figure 21-28 represents a closed cycle for a gas (the figure is not dr...
Text Solution
|
- Calculate the specific heat of a metal from the following data. A cont...
Text Solution
|
- When 0.40 mol of oxygen (O2) gas is heated at constant pressure starti...
Text Solution
|
- A gas is to be expanded from initial state i to final state f along ei...
Text Solution
|
- When 22.5 J was added as heat to a particular ideal gas, the volume of...
Text Solution
|
- Suppose 2.80 mol of an ideal diatomic gas, with molecular rotation but...
Text Solution
|
- The temperature of 4.50 mol of an ideal monatomic gas is raised 27.0 K...
Text Solution
|
- Opening champagne. In a bottle of champagne, the pocket of gas (primar...
Text Solution
|
- One mole of an ideal diatomic gas goes from a to c along the diagonal ...
Text Solution
|
- Adiabatic wind. The normal airflow over the Rocky Mountains is west to...
Text Solution
|
- We give 90 J as heat to a diatomic gas, which then expands at constant...
Text Solution
|
- Suppose 0.825 mol of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- Figure 21-31 shows a cycle undergone by 2.00 mol of an ideal monatomic...
Text Solution
|
- An ideal diatomic gas, with rotation but no oscillation,undergoes an a...
Text Solution
|
- The volume of an ideal gas is adiabatically reduced from 350 L to 130 ...
Text Solution
|
- Under constant pressure, the temperature of 3.00 mol of an ideal monat...
Text Solution
|
- An automobile tire has a volume of 1.64 xx 10^2 m^3 and contains air ...
Text Solution
|
- Suppose 1.00 L of a gas with gamma= 1.30, initially at 285 K and 1.00 ...
Text Solution
|