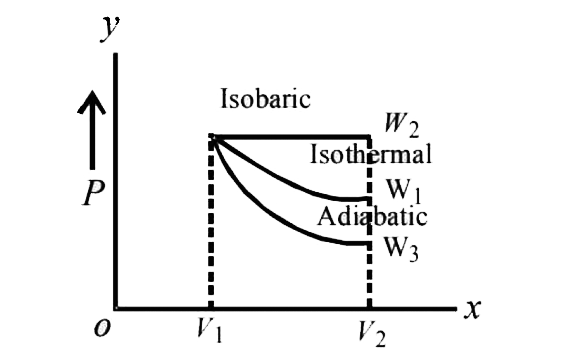
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( MORE THAN ONE CORRECT CHOICE TYPE )|9 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( LINKED COMPREHENSION )|15 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PROBLEMS|41 VideosTEMPERATURE, ZEROTH LAW OF THERMODYNAMICS AND THERMAL EXPANSION
RESNICK AND HALLIDAY|Exercise PRACTICE QUETIONS (Integer Type)|4 VideosTHE KINETIC THEORY OF GASES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS|72 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-THE FIRST LAW OF THERMODYNAMICS-PRACTICE QUESTIONS ( SINGLE CORRECT CHOICE TYPE )
- A thermally isolated sample of an ideal gas at a fixed temperature is ...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- Variation of internal energy with density of 1 "mole" of monatomic gas...
Text Solution
|
- The pressure P, Volume V and temperature T of a gas in the jar A and t...
Text Solution
|
- A Carnot engine operates between hot and cold reservoirs with temperat...
Text Solution
|
- In an adiabatic change, the pressure and temperature of a monoatomic g...
Text Solution
|
- Neon is a monatomic gas with a molar heat capacity at constant volume ...
Text Solution
|
- A cylindrical chamber A of uniform cross section is divided into two p...
Text Solution
|
- Determine the quantity of heat added to 3.5 moles of the ideal...
Text Solution
|
- 175 calories of heat is reuired to raise the temperature of 5 m...
Text Solution
|
- If one complete cycle of a reversible process is carried out o...
Text Solution
|
- The efficiency of a heat engine working between the freezing ...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- An ideal monatomic gas undergoes an adiabatic process , and its ...
Text Solution
|
- An enegine is used to lift a 2700 kg truck to a height of 3.0...
Text Solution
|
- The slope of isothermal and adiabatic curves are related as
Text Solution
|
- Two moles of a confined ideal monatomic gas begin at state A in the pr...
Text Solution
|
- Pressure vs. volume graphs for a certain gas undergoing five different...
Text Solution
|
- In an adiabatic process
Text Solution
|