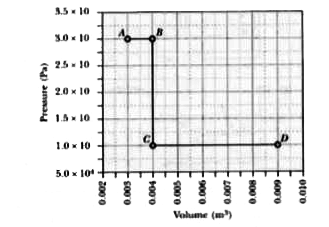
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( MORE THAN ONE CORRECT CHOICE TYPE )|9 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS ( LINKED COMPREHENSION )|15 VideosTHE FIRST LAW OF THERMODYNAMICS
RESNICK AND HALLIDAY|Exercise PROBLEMS|41 VideosTEMPERATURE, ZEROTH LAW OF THERMODYNAMICS AND THERMAL EXPANSION
RESNICK AND HALLIDAY|Exercise PRACTICE QUETIONS (Integer Type)|4 VideosTHE KINETIC THEORY OF GASES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS|72 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-THE FIRST LAW OF THERMODYNAMICS-PRACTICE QUESTIONS ( SINGLE CORRECT CHOICE TYPE )
- 175 calories of heat is reuired to raise the temperature of 5 m...
Text Solution
|
- If one complete cycle of a reversible process is carried out o...
Text Solution
|
- The efficiency of a heat engine working between the freezing ...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- An ideal monatomic gas undergoes an adiabatic process , and its ...
Text Solution
|
- An enegine is used to lift a 2700 kg truck to a height of 3.0...
Text Solution
|
- The slope of isothermal and adiabatic curves are related as
Text Solution
|
- Two moles of a confined ideal monatomic gas begin at state A in the pr...
Text Solution
|
- Pressure vs. volume graphs for a certain gas undergoing five different...
Text Solution
|
- In an adiabatic process
Text Solution
|
- A jogger's internal energy changes because he performs 6.4 xx 10^5 J...
Text Solution
|
- The pressure and volume of a gas are changed along the path ABCA. Usin...
Text Solution
|
- The temperature of a monatomic ideal gas remains constant during a pro...
Text Solution
|
- Heat is added isothermally to 2.5 mol of a monatomic ideal gas. The te...
Text Solution
|
- One mole of a monatomic ideal gas has an initial pressure, volume, and...
Text Solution
|
- Suppose a monatomic ideal gas is contained within a vertical cylinder ...
Text Solution
|
- The efficiency of an automobile engine increases by 5.0%. For an input...
Text Solution
|
- Engine 1 has an efficiency of 0.18 and requires 5500 J of input heat t...
Text Solution
|
- A Carnot engine has an efficiency of 0.40. The Kelvin temperature of i...
Text Solution
|
- The hot reservoir for a Carnot engine has a temperature of 890 K, whil...
Text Solution
|