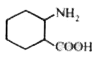
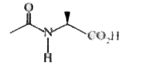
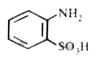
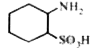
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder ( Topicwise ) (Carbohydrates and Lipids )|23 VideosBIOMOLECULES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder ( Topicwise ) (Amino Acids , Proteins and Enzymes )|21 VideosAMINES
DISHA PUBLICATION|Exercise Exercise - 2 : Concept Applicator|30 VideosCHAPTERWISE NUMERIC/INTEGER ANSWER QUESTIONS
DISHA PUBLICATION|Exercise CHAPTER 28- BIOMOLECULES|10 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-BIOMOLECULES -Exercise - 2 : Concept Applicator
- Which of the following will not exist in zwitter ionic form at pH=7?
Text Solution
|
- Vitamin D is also known as
Text Solution
|
- Which of the following pairs of compounds can be distinguished by usin...
Text Solution
|
- Which of the following carbohydrates is not related to (+)-glucose?
Text Solution
|
- Number of possible stereoisomers of glucose and fructose.
Text Solution
|
- For the complex conversion of D-glucose into the corresponding osazone...
Text Solution
|
- Which reagents can be used to distinguish glucose and fructose ? (I)...
Text Solution
|
- Among the three compounds shown below, two yield the same product on r...
Text Solution
|
- A distinctive and characteristic functional group of fat is
Text Solution
|
- Iso-electric point of alanine is (pH =6). At which pH, maximum concent...
Text Solution
|
- Find iso-electric point of given amino acid CH(3)-underset(o+)unders...
Text Solution
|
- A biological catalyst is essentially
Text Solution
|
- In an amino acid, the carboxyl group ionises at pK(a1) = 2.34 and ammo...
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Among the following vitamins the one whose deficiency causes rickets (...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following is known as the universal energy currency of th...
Text Solution
|
- The base present in DNA, but not in RNA is
Text Solution
|
- Double stranded DNA virus with 20,000 base pairs has nucleotides
Text Solution
|
- Fructose reduces Tollens' reagent due to :
Text Solution
|
- Which one of the following statements is not true regarding ( +) Lacto...
Text Solution
|