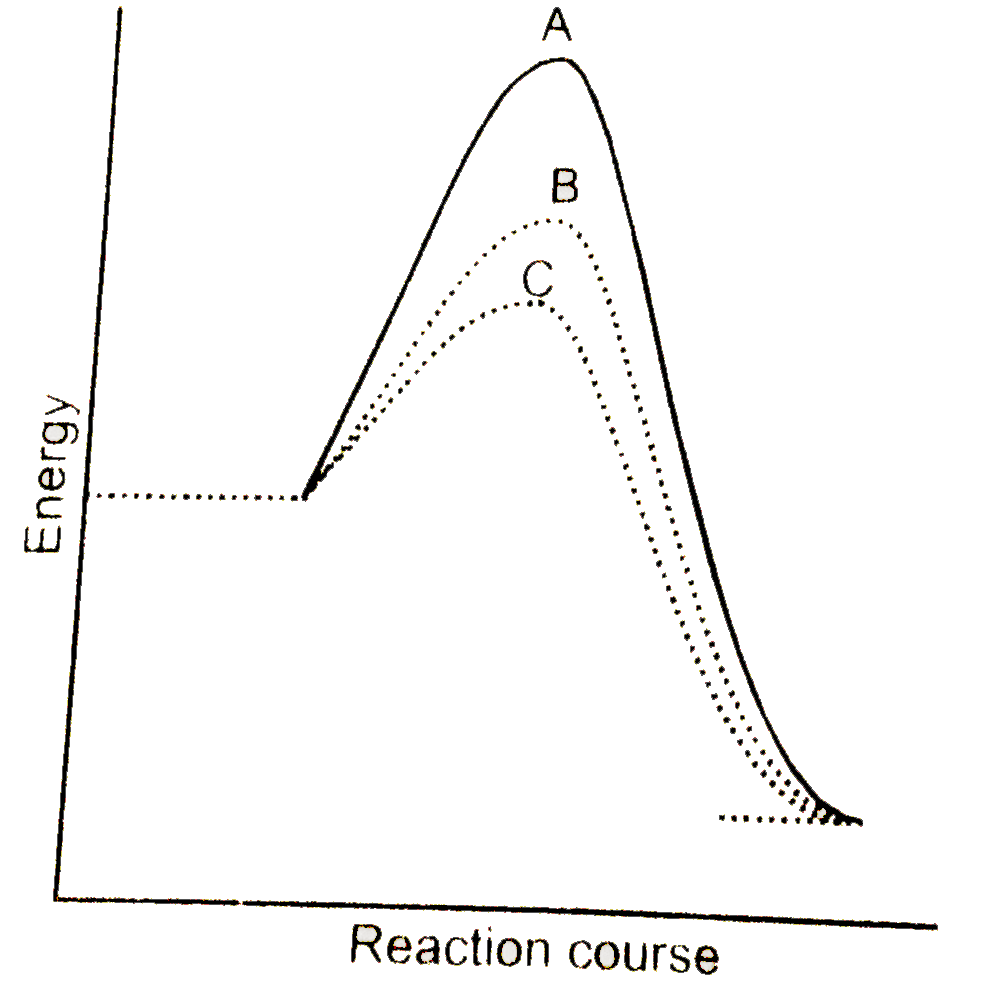
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 2 : CONCEPT APPLICATOR|30 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 1 : CONCEPT BUILDER (TOPICWISE) (TOPIC 2: Order of Reaction and Half Life Period)|32 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-2: CONCEPT APPLICATOR|30 VideosCHEMISTRY IN EVERDAY LIFE
DISHA PUBLICATION|Exercise Exercise|88 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMICAL KINETICS -EXERCISE 1 : CONCEPT BUILDER (TOPICWISE)(TOPIC 3 : Theories of Rate of Reaction)
- Rate constant k = 1.2 xx 10^(3) mol^(-1) L s^(-1) and E(a) = 2.0 xx 10...
Text Solution
|
- An exothermic reaction ArarrB has an activation energy of 17 kJ per mo...
Text Solution
|
- A catalyst only
Text Solution
|
- An example of autocatalytic reaction is
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- The activation energies of the forward and backward reactions in the c...
Text Solution
|
- A homogenous catalytic reaction takes place through the three alternat...
Text Solution
|
- For an exothermic reaqction, the energy of activation of the reactants...
Text Solution
|
- The activation energy for a simple chemical reaction A rarr B is E(a) ...
Text Solution
|
- For a reaction the activation energy Ea=0 and the rate constant=3.2 ti...
Text Solution
|
- A graph plotted between log k versus 1//T for calculating activation e...
Text Solution
|
- When a biochemical reaction is carried out in laboratory from outside ...
Text Solution
|
- For an endothermic reaction, energy of activation is Ea and enthalpy o...
Text Solution
|
- In a zero-order reaction for every 10^(@) rise of temperature, the rat...
Text Solution
|
- Activation energy (E(a)) and rate constants (k(1) and k(2)) of a chemi...
Text Solution
|
- What is the activation energy for a reaction if its rate doubles when ...
Text Solution
|
- The activation energy of a reaction can be determined from the slope o...
Text Solution
|