A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE(ADVANCE) FOR IIT ENTRANCE)|5 VideosSOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|10 VideosSOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)|18 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE(MAIN) & OTHER STATE BOARDS ENGINEERING ENTRANCE)
- The contribution of a particle at the edge centre of a particular unit...
Text Solution
|
- Which of the following compounds is metallic and ferromagnetic ?
Text Solution
|
- The corrent statement regarding defects is solids in solids is
Text Solution
|
- In a face centred cubic arrangement of A and B atoms whose A atoms are...
Text Solution
|
- A metal crystallises in a face centred cubic structure. If the edge le...
Text Solution
|
- Edge length of cube is 300 pm. Its body diagonal would be:
Text Solution
|
- An example of a non-stoichiometric oxide when heated is
Text Solution
|
- A given metal crystallises out with a cubic structure having edge leng...
Text Solution
|
- The number of atoms in 2.4 g of body centred cubic crystal with edge l...
Text Solution
|
- Which among the following statement is true about Schottky defect ?
Text Solution
|
- If a metal crystallises in bcc structure with edge length of unit cell...
Text Solution
|
- At 100^(@)C, copper (Cu) has FCC unit cell structure will cell edge le...
Text Solution
|
- Which premitive unit cell has unequal edge length (a != b != c) and al...
Text Solution
|
- A compound of formula A(2)B(3) has the hcp lattice. Which atom forms t...
Text Solution
|
- A solid having density of 9 xx 10^(3) kg m^(-3) forms face centred cub...
Text Solution
|
- The radius of the largest shpere which fits properly at the centre of ...
Text Solution
|
- Element 'B' forms ccp structure and 'A' occupies half of the octahedra...
Text Solution
|
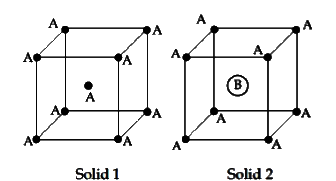
- Consider the bcc unit cells of the solids 1 and 2 with the position of...
Text Solution
|
- The ratio of number of atoms present in a simple cubic body centred cu...
Text Solution
|
- An element has a face-centred cubic (fcc) structure with a cell edge o...
Text Solution
|